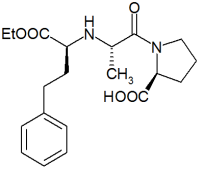
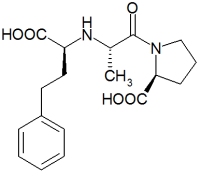
Enalapril maleate is an orally active angiotensin converting enzyme inhibitor. It lowers peripheral vascular resistance without causing an increase in heart rate. Enalapril administered in doses of 10 to 40 mg/d is effective in lowering blood pressure in all grades of essential and renovascular hypertension. In patients with severe congestive heart failure that is resistant to conventional therapy, enalapril appears to be well tolerated, with few serious adverse effects reported.(1)
The most frequently reported side effects during clinical trials with enalapril include headache, dizziness, fatigue, diarrhea, nausea, rash, cough, hypotension, and angioneurotic edema. The majority of these effects were mild, transient, and probably unrelated to treatment as they were seen with similar frequencies in patients receiving placebo. Serious adverse effects with enalapril have been rare. Reversible deterioration of renal function has been reported in a few patients and appears more frequently if hydrochlorothiazide is given concurrently with enalapril. (1)
Elevation of liver enzymes has been reported in a few patients,(1) but a definite causal relationship to enalapril therapy has rarely been established.(2-3)
We describe a patient in whom cholestatic-llkc disturbances of liver function tests were detected shortly after initiating therapy with enalapril. We confirmed this by testing the immunosensitivity of the patient to the drug. Case Report A 59-year-old female patient, who had been diagnosed previously as suffering from hypertension, was found to be noncompliant with her drug therapy and her blood pressure follow-up. Apart from hypertension, she was in good health. She recently appeared in our clinic for a routine examination, and was found to have an elevated blood pressure of 200/105 nun Hg in repeated measurements. her physical examination revealed no other pathological findings.
We recommended treatment with enalapril, 20 mg once daily. She was seen 2 months later, and her blood pressure was found to be 180/90 mm Hg.
Laboratory tests showed a raised alkaline phosphatase of 305 IU (normal being up to 210 IU) and a raised scrum gamma-glutamyl transpeptidase of 95 IU/L (normal up to 25 IU/L). Serum levels of proteins, bilirubin and glutamic-oxaloacetic transaminase (GOT) were normal. The erythrocyte sedimentation rate was 42 mm in the 1st hour, and a complete blood count was normal. Scrum antinuclear factor was not found, and the latex test was negative. Serum Australia antigen was not found, nor were antibodies to smooth muscle, microsomes, or mitochondria. A technctium scan of the liver and spleen was normal. An ultrasound examination of the gallbladder, pancreas, and bile ducts was normal. Macrophage migration inhibition factor in the patient's blood in the presence of enalapril was positive. We also found scrum immunoglobulin E antibodies to enalapril in the patient's serum by finding degranulation of basophil cells in the presence of enalapril. (Livni et al(4) have described the immunologic laboratory tests.) In the light of the laboratory tests, we decided to cease enalapril therapy in our patient. Two months later her blood pressure was found to be 140/80 mm Hg, and the serum levels of alkaline phosphatase and gamma glutamyl transdase peptidase reverted to normal. Discussion In the case described we found disturbed liver function test of a cholestatic nature (raised serum alkaline phosphatase and gamma glutamyl transpeptidase), which appeared shortly after initiating therapy with enalapril. Further investigation ruled out the presence of gallstones, primary biliary cirrhosis, or tumors of the gallbladder, pancreas, or liver. The disappearance of the cholestatic signs after the discontinuation of enalapril, as well as the laboratory tests that proved immunologic sensitization of the humoral and cellular systems to enalapril, clearly indicated that the drug enalapril was the cause of impaired liver function tests in this case.
Because of the positive immunologic tests and the normal blood pressure of the patient after the cessation of treatment, we found it unnecessary to reinitiate enalapril therapy for the purpose of causing a recurrence of impaired liver function.
Drug-inducede hepatoxicity can resemble any form of chronic or acute liver dysfunction, including hepatoc-cellular necrosis, cholestasis, and a combination of the two. The liver is particularly susceptible to drug-induced injury because of its blood supply and its role in drug metabolism and biotransformation.(5-8)
Cholestasis secondary to drugs can cause a dilemma in the differential diagnosis of jaundice. According to one study,(9) drug hepatotoxicity may be a factor in 2% to 5% of jaundiced patients. In the elderly this figure may be as high as 20%.(10)
Drugs rarely seem to cause damage by direct action on the liver cells, and two other mechanisms are probably involved. The first is mediated by metabolite-related substances, which combine covalently with cell proteins. the second is by the development of an immunologic reaction to the drug, which renders a constituent of the liver cell antigenic. In the case of some drugs, such as paracetamol (acetaminophen), the metabolite-related injury seems particularly important. In others, for instance, halothane, the immunologic reaction may be predominant.(7)
Drug-induced cholestasis is usually due to an alteration in the liver's ability to secrete bile. Almost all classes of medications include agents that may cause cholestasis, but the mechanisms of action may differ. For example, cholestasis may be caused by a change in the chemical and physical properties of the hepatocytic membranes, as with estrogens, or by an associated allergic or autoimmune attachment to the hepatocyte or the microscopic bile ducts, as with phenothiazines and related antipsychotic agents, or by a sclerosing cholangitis of the intermediate and large bile ducts, as with intra-arterial infusion of floxuridine.(6)
After the description of our case, enalapril must be added to the list of drugs that may cause cholestatic-like impaired liver function tests in those patients ingesting the drug. Any patient developing impaired liver function tests while being treated with this drug would be well advised to cease taking enalapril as a first step in elucidating the cause of this impairment.
Key words. Enalapril; liver function tests; immunologic tests. References 1. Todd PA, Heel RC. Enalapril-a review of its pharmacodynamic
and pharmacokinetic properties and therapeutic use in hypertension
and congestive heart failure. Drugs 1981; 31:198. 2. Mikloweit P, Bienmuller H. Drug-induced intrahepatic cholestasis
caused by flecainide acetate and enalapril. Internist (Berlin) 1987;
28:193. 3. Lunel F, Grippon P, Cadranel GF, et al. Acute hepatitis after
taking enalapril maleate. Gastroenterol Clin Biol 1987; 11:174. 4. Livni E, Halevy S, Stahl B, et al. The appearance of macrophage
migration-inhibition factor in drug reactions. J Allergy Clin Immunol
1987; 80:843. 5. Ludwig G, Axelscn R. Drug effects on the liver. Dig Dis Sci 1983;
28:651. 6. Kaplowitz N, Yee-Aw T, Simmon FR, ct al. Drug-induccd
hepatoxicity. Ann Intern Med 1986; 104:826. 7. Progress report, hepatic reactions to drugs. Gut 1979; 20:634. 8. Zimmerman HG, Lewis GH. Drug-induced cholestasis. Med Toxicol
1987; 2:112. 9. Koff RS. Profile of hyperbilirubinemia in the hospital population.
Clin Res 1970; 18:680. 10. Eastwood HO. Causes of jaundice in the elderly: a survey of
diagnosis and investigation. Gerontol Clin 1971; 13:69. Submitted, revised, May 24, 1991. From the Kupat Holim Clinics and Department of Family Medicine, Tel Aviv University, Israel. Requests for reprints should be addressed to Simon Zalewski, MD, Moshav Herut, Tel Mond 40691, Israel. [C] 1991 Appleton & Lange ISSN 0094-3509
COPYRIGHT 1991 Dowden Health Media, Inc.
COPYRIGHT 2004 Gale Group