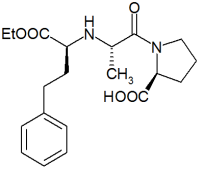
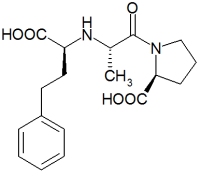
We evaluated the safety of enalapril administration in 20 very old (76 [+ or -] 7 years) patients with rapidly progressive congestive heart failure (deterioration from New York Heart Association class 2 to class 4 on admission). They were all given increasing doses of enalapril regardless of concomitant diuretic therapy and state of hydration. Renal function deteriorated in four patients (group A) and remained unchanged in 16 (group B). The mean pretreatment serum creatinine level in group A was significantly higher than that in group B (2.4 vs. 1.3 mg/dl, p<0.001). No patient with a serum creatinine level less than 1.9 mg/dl on admission had further impairment of renal function. Groups A and B did not differ by age, concomitant diseases (including hypertension and diabetes mellitus), or medications (including diuretics) or by in-hospital serum electrolyte concentrations and blood pressure. Renal damage was noted during the initial four days of the study and was reversible following discontinuation of enalapril. Our data suggest that enalapril can be safely administered to very old patients with rapidly progressive congestive heart failure provided that the initial serum creatinine level is below 1.9 mg/dl. In patients with a higher serum creatinine level, careful monitoring and prompt discontinuation of enalapril administration can prevent irreversible renal damage. (Chest 1991; 100:1558-61)
ACE = angiotensin converting enzyme; CHF = congestive heart failure; NYHA = New York Heart Association
The efficacy of angiotensin converting enzyme (ACE) inhibition in the treatment of congestive heart failure (CHF) has been well established.[1-4] However, this modality has opposing effects on renal hemodynamics, namely: reduction in systemic vascular resistance, which may either decrease or increase renal blood flow, and vasodilatation of the efferent glomerular arterioles, which results in decreased glomerular perfusion pressure.[5,6]
Several studies have focused on changes in renal function following the administration of ACE inhibitors to patients with CHF.[7-9] Several risk factors predisposing to deterioration of renal function have been documented: severe heart failure, hyponatremia, prolonged hypotensive effect of the drug, high doses of concomitant diuretics, and diabetes mellitus.[7-9] In view of these factors, it is currently recommended that prior to initiation of ACE inhibitors, diuretic therapy should be discontinued, and patients should be well hydrated. This recommendation is often impractical and may prevent or delay the administration of ACE inhibitors to acutely ill patients admitted because of severe deterioration of cardiac function. In addition, the safety of these drugs in elderly patients has not been established.
In this study, we evaluated the renal toxicity of enalapril following its administration to very old patients with severe CHF, regardless of their state of hydration or concomitant diuretic therapy.
Patients and Methods
The study population included 20 patients aged 70 years or older who were admitted to the hospital for rapid deterioration of cardiac function (from class 2 to class 4 in the New York Heart Association [NYHA] classification). Exclusion criteria included prior treatment with vasodilators or salt-restriction diet, serum, creatinine level above 3 mg/dl, serum potassium level above 5.5 mEq/L, and systolic blood pressure under 100 mm Hg. Patients were to receive gradually increasing doses of enalapril starting with 4 mg on day 1, with daily increments of 5 mg up to 20 mg or until satisfactory therapeutic response had been achieved. Laboratory and clinical assessment was performed prior to drug administration and on days 2, 4, and 30 after admission. An increase in serum creatinine concentration of more than 0.7 mg/dl above the baseline level was an indication for discontinuation of the drug. This value was chosen for being equal to 2 SD of the mean change in serum creatinine level observed over the same follow-up period in a control group of six patients who were not receiving vasodilators.
Results
The mean age of the study group was 75.6 [+ or -] 6.7 years (Table 1). There were 14 men and 6 women. Nine had hypertension, and five were diabetic. Heart failure resulted from ischemic cardiomyopathy in all patients who were classified as NYHA class 2 prior to admission. All patients were admitted following rapid escalation of CHF; however, none had any evidence of acute myocardial infarction. All patients were receiving furosemide, 40 to 80 mg/day. This dose was maintained, and no medications other than enalapril were added throughout the study period. Enalapril was discontinued in four patients (group A) because of deterioration of renal function. The other 16 patients (group B) were receiving 20 mg of the drug by day 4 and maintained this dosage for the rest of the study.
Nine patients had a pretreatment serum creatinine level above 1.4 mg/dl. Administration of enalapril was discontinued in four patients (group A) following a significant rise (>0.7 mg/dl) in creatinine concentration. Three additional patients showed mild changes (<0.4 mg/dl) in creatinine level, and the remaining two patients had an early rise in serum creatinine during the first four days, with partial or complete reversal during the follow-up period. The mean increase in serum creatinine level in group A was
0.85 [+ or -] 0.13 mg/dl, compared with 0.01 [+ or -] 0.2 mg/dl
in the remaining 16 patients (p<0.0001). A parallel increase was observed in mean blood urea nitrogen level (12.5 [+ or -] 10.3 vs. 0.14 [+ or -] 4.8 mg/dl, p = 0.003). The mean pretreatment serum creatinine level in group A patients was significantly higher than that in group B (2.38 [+ or -] 0.3 vs. 1.32 [+ or -] 0.18 mg/dl, p<0.001). In fact, all group A patients had an initial serum creatinine level equal to or above 1.9 mg/dl. [TABULAR DATA 1 OMITTED]
Patients in group A and B were similar with respect to age and disease profile, including past history of hypertension and diabetes mellitus, and did not differ as to concomitant medications (including furosemide) or pretreatment mean arterial pressure. During the study, the mean arterial pressure decreased in both groups to the same degree ( - 13 [+ or -] 26 vs - 9 [+ or -] 10 mm Hg, p>0.5). None of the 20 patients developed significant electrolyte abnormalities during the study and the follow-up period. However, group A patients had a higher pretreatment mean serum sodium concentration (143 [+ or -] 3.5 vs 138 [+ or -] 2.17 mEq/L; p<0.003). During the study period, the serum sodium level tended to decrease in group A patients ( - 3.5 [+ or -] 3.1 mEq/L) but increased slightly in group B patients (1.3 [+ or -] 3.3 mEq/L).
Of the four patients with an initial serum creatinine level of more than 1.9 mg/dl, three exhibited further deterioration of renal function following the administration of enalapril. The dose of enalapril was changed to 5 mg in one patient and 10 mg in the other two. In all four patients, deterioration was observed during the first four days of therapy, necessitating prompt discontinuation of the drug. This was followed by complete restoration of initial renal function within three to five days.
With regard to their clinical condition, all 20 patients greatly improved during their hospitalization, which lasted on average 4.4 [+ or -] 0.4 days, and maintained that improvement throughout the 30-day follow-up period.
Discussion
The beneficial effects of ACE inhibitors in CHF are well established and include improved hemodynamics, alleviation of symptoms, and increased survival.[1-4] Recent studies have suggested that ACE inhibitors can decease progression of renal failure in diabetic patients[10-12] and improve renal function in patients with systemic lupus erythematosus.[13] Patients with CHF, however, have remained a subgroup in which ACE inhibitors might have a deleterious effect on renal function. A recent study[7] suggested that activation of the renin-angiotensin system in patients with CHF serves mainly to preserve renal function rather than to maintain sodium volume or blood pressure. Angiotensin II specifically constricts the efferent glomerular arterioles,[5,6] thus increasing net intraglomerular pressure and maintaining the filtration rate, which otherwise would decrease because of a fall in cardiac output and an increase in peripheral resistance.
Management of CHF could be greatly improved if one could predict renal deterioration induced by ACE inhibitors prior to their administration. The following have been suggested as potential risk factors: compromised renal perfusion as evidenced by hyponatremia,[9,14] sodium depletion usually due to diuretic therapy,[8] diabetes mellitus,[8] and the prolonged hypotensive effect of long-acting ACE inhibitors.[15] In view of these factors, it has been recommended that, prior to the administration of ACE inhibitors, all other diuretics should be discontinued and the state of hydration should be established. This recommendation is impractical in acutely ill patients who might benefit from the early administration of ACE inhibitors concurrent with ongoing diuretic therapy.
Many studies have emphasized the age-dependent decline in renal function often potentiated by drugs such as nonsteroidal anti-inflammatory agents.[16] However, as yet no study has referred to age per se as a potential risk factor for ACE inhibitor-induced deterioration of renal function, especially in patients with CHF. The ever-increasing age and severity of CHF in hospitalized patients necessitates the search for additional therapeutic modalities, especially when the usual diuretic therapy has been exhausted.
In general, the therapeutic enalapril regimen used by us proved to be extremely safe in spite of the rapidly escalating dose regimen. Of the 20 very old patients (mean age, 76 years), 16 (group B) had no evidence of deteriorating renal function. Only four patients (group A) had a decrease in renal function as evidenced by a significant increase in serum creatinine concentration (>0.7 mg/dl). Discontinuation of the drug was accompanied by rapid restoration of pretreatment renal function in all patients.
One should mention that none of our group A patients had any of the recognized risk factors for ACE inhibitor-induced renal impairment, namely: hyponatremia, massive diuretic therapy, or hypotension. The only predictive risk factor in these four patients was a pretreatment serum creatinine level of more than 1.9 mg/dl. Of the 20 patients included in the present study, there was only one with an initial serum creatinine level of more than 1.9 mg/dl whose renal function remained unchanged. None of the patients who entered the study with a serum creatinine level below 1.9 mg/dl had further impairment of renal function. Interestingly, pretreatment serum creatinine level has not been previously mentioned as one of the predictors of renal toxicity due to ACE inhibitors.[8]
Further studies in patients with serum creatinine levels above 1.9 mg/dl are needed to determine whether use of very low doses of enalapril (eg, 2.5 mg) will result in a significant decrease in renal toxicity while maintaining drug effectiveness. In spite of the proved safety of enalapril in our study population, four (20 percent) patients developed significant renal toxicity. What is the possible underlying mechanism for this toxicity? The clearance of enalapril is primarily renal and is much slower in patients with CHF.[17,18] We predicted longer duration of action of the drug in these four patients and therefore limited the dosage of enalapril to 10 mg/day; however, even this dose still resulted in marked renal impairment.
One could speculate that some of our selected group of elderly patients with probably advanced atherosclerosis had bilateral renal artery stenosis. This condition, in which the glomerular capillary hydraulic pressure is maintained predominantly by an angiotensin-mediated increase in efferent arteriolar tone, has been previously associated with a deleterious renal effect of ACE inhibitors.[19] Hence, our group A patients might have suffered from significant bilateral renal stenosis. The contribution of this condition to renal toxicity induced by ACE inhibitors should be further explored in studies in which changes in renal function during ACE inhibitor therapy are correlated with the degree of anatomic and functional stenosis of both renal arteries.[20]
In conclusion, our study suggests that ACE inhibitors, even long-acting ones, such as enalapril, can be safely administered to very old patients with severe CHF. These agents can be given immediately on admission, in rapidly increasing doses, without prior discontinuation of diuretics or rehydration, as long as the initial serum creatinine level is less than 1.9 mg/dl. Furthermore, ACE inhibitors also can be administered to patients with a serum creatinine level of more than 1.9 mg/dl provided that their renal function is closely monitored. Prompt discontinuation of the drug in the presence of a progressive increase in serum creatinine level usually results in rapid restoration of renal function.
References
[1] Kramer BL, Massie BM, Topic N. Controlled trial of captopril in chronic heart failure: a rest and exercise hemodynamic study. Circulation 1983; 67:807-16 [2] Sharpe DN, Murphy J, Coxon R, Hannan SF. Enalapril in patients with chronic heart failure: a placebo-controlled, randomized, double blind study. Circulation 1984; 70:271-78 [3] Franciosa JA, Wilen MM, Jordan RA. Effects of enalapril, a new angiotensin converting enzyme inhibitor, in a controlled trial on heart failure. J Am Coll Cardiol 1985; 5:101-07 [4] CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med 1987; 316:1429-435 [5] Ichikawa I, Brenner BM. Glomerular action of angiotensin 2. Am J Med 1984; 76:43-9 [6] Packer M. Interaction of prostaglandins and angiotensin 2 in the modulation of renal function in congestive heart failure. Circulation 1988; 77(suppl 1):64-73 [7] Packer M. Why do the kidneys release renin in patient with congestive heart failure? a nephrocentric view of converting enzyme inhibition. Am J Cardiol 1987; 60:179-84 [8] Packer M, Lee WH, Medina N, Yushac RN, Kessler PD. Functional renal insufficiency during long term therapy with captopril and enalapril in severe chronic heart failure. Ann Intern Med 1987; 106:346-54 [9] Packer M, Lee WH, Kessler PD, Medina N, Yushac M, Gottlieb SS. Identification of hyponatremia as a risk factor for the development of functional renal insufficiency during converting enzyme inhibition in congestive heart failure. J Am Coll Cardiol 1987; 10:837-44 [10] Bjork S, Nyberg G, Mulec H, Granerus G, Heritz H, Aurell M. Beneficial effects of angiotensin converting enzyme inhibition on renal function in patient with diabetic nephropathy. Br Med J 1986; 293:467-70 [11] Abraham PA, Opsahl JA, Halstenson CE, Keane WF. Efficacy and renal effects of enalapril therapy for hypertensive patients with chronic renal insufficiency. Arch Intern Med 1988; 148:2358-362 [12] Bear R. Enalapril therapy in patients with renal function impairment. Arch Intern Med 1988; 148:2343-344 [13] Herlitz H, Edeno C, Mulec H, Westberg G, Aurell M. Captopril treatment of hypertension and renal failure in systemic lupus erythematosus. Nephron 1984; 38:253-56 [14] Lilly LS, Dzau VJ, Williams GH, Rydstedt L, Hollenberg NK. Hyponatremia in congestive heart failure: implication for neurohormonal activation and responses to orthostasis. J Clin Endocrinol Metab 1984; 59:924-30 [15] Packer M, Lee WH, Yushak M, Medina RN. Comparison of captopril and enalapril in patients with severe congestive heart failure. N Engl J Med 1986; 315:847-53 [16] Dahl SL, Ward JR. Efficacy and tolerability of oxaprozin in the elderly. Semin Arthritis Rheum 1986; 15(suppl 2):40-6 [17] Kubo SH, Cody RJ. Clinical pharmacokinetics of the angiotensin converting enzyme inhibitors. Clin Pharmacokinet 1985; 10:377-91 [18] Dickstein M, Till AE, Aarsland T, Tzelta K, Abrahamsen AM, Kristiansen K, et al. The pharmacokinetics of enalapril in hospitalized patients with congestive heart failure. Br J Clin Pharmacol 1987; 23:403-10 [19] Hricik DE, Browning PJ, Kopelman R, Goorno WE, Madias NE, Dzau VJ. Captopril induced functional renal insufficiency in patients with bilateral renal artery stenosis or renal artery stenosis in a solitary kidney. N Engl J Med 1983; 308:373-76 [20] Salmon P, Brown MA. Renal artery stenosis and peripheral vascular disease: implication for ACE inhibitor therapy (letter). Lancet 1990; 336:321
Doron Schwartz, M.D; Mordechai Averbuch, M.D.; Amos Pines, M.D.; Ran Kornowski, M.D.; and Yoram Levo, M.D. From the Department of Medicine "T," Ichilov Hospital, Tel-Aviv Sourasky Medical Center, and the Sackler Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
COPYRIGHT 1991 American College of Chest Physicians
COPYRIGHT 2004 Gale Group