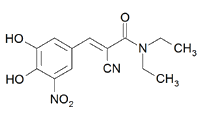
The FDA approved Novartis' entacapone (Comtan), a new drug for Parkinson's disease. The new drug belongs to a class of drugs known as COMT inhibitors, which enhance the benefits of levodopa by reducing its breakdown prior to reaching the brain. Entacapone is the second COMT inhibitor to be approved.
Entacapone has a favorable adverse effect profile and was generally well tolerated in clinical trials, according to Novartis. Although the drug's labeling does not require specific safety monitoring, the labeling states that cases of severe rhabdomyolysis have been reported (F-D-C Reports. 1999;October 25:3) and that it is impossible to determine what role, if any, entacapone played in their pathogenesis. The most commonly reported adverse effects were dyskinesia, nausea, diarrhea, abdominal pain, and urine discoloration.
Copyright Springhouse Corporation Feb 2000
Provided by ProQuest Information and Learning Company. All rights Reserved