Please note: the events and trends listed on this timeline are by year only and are not listed in any particular order within each year. The sources of information used for this timeline include CFA publications, public record, peer-reviewed literature and conference proceedings, and Internet-based timelines from AEGIS (aegis.org), Kaiser Family Foundation (kff.org), and Gay Men's Health Crisis (gmhc.org). Not all events, deaths, or data could be included in this timeline.
1995
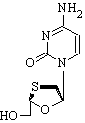
* The FDA approves the nucleoside reverse transcriptase inhibitor (NRTI), lamivudine (Epivir, 3TC), by accelerated approval.
* December 6, 1995: the FDA approves saquinavir (Invirase) in a record 97 days. This is the first antiretroviral drug in the protease inhibitor (PI) class indicated for the treatment of HIV disease. This is essentially the beginning of HAART--Highly Active Anti-Retroviral Therapy.
APPROVED
* The FDA approves the chemotherapy agent doxorubicin liposome injection (Doxil) for the treatment of Kaposi's sarcoma (KS) in patients with AIDS whose disease has progressed on prior chemotherapy or in patients who cannot tolerate these other chemotherapy agents.
* The US Public Health Service (USPHS) and Infectious Diseases Society of America (IDSA) publishes the USPHS/IDSA Guidelines for the Prevention of Opportunistic Infections in Persons Infected with HIV on July 14, 1995.
* US acknowledges role played by the Institut Pasteur (France) in co-discovering HIV as the virus that causes AIDS.
* 2nd National Conference on Human Retroviruses and Related Infections is held in Washington, DC.
* Founding of the International Association of Physicians in AIDS Care (IAPAC).
AIDS
* The Centers for Disease Control (CDC) announces that AIDS has become the leading cause of death tot Americans aged 25 to 44. The biggest increase is reported among men of color who have sex with men.
* President Clinton establishes Presidential Advisory Council on HIV/AIDS. The First White House Conference on HIV/AIDS is held.
* Founding of The Center for AIDS: Hope & Remembrance Project (CFA), a dba of AIDS Research Consortium of Houston.
* In 1995, the CDC reports new cases of AIDS reach 74,180 in the US and the cumulative total number of AIDS cases in the US is 513,486. However, tracking of HIV infections is still not widely instituted.
* First issue of Research Initiative/Treatment Action! (RITA!) is published and includes coverage of a paper published on March 3 in The New England Journal of Medicine: the use of interleukin-2 (IL-2) in 10 patients with HIV. Six patients experienced increases in CD4 counts.
* The RITA! Fax Newsletter (later renamed in 2000 as the RITA! Weekly Newsletter) is launched as a convenient information product for receiving weekly news on HIV treatment and research (more information: centerforaids.org/rita/weekly.htm).
* AIDS patient receives baboon bone marrow cells in an effort to boost his immune system, a strategy that ultimately did not prove successful.
1996
* US Congress reauthorizes the Ryan White CARE Act.
* The FDA approves the PIs ritonavir (Norvir) and indinavir (Crixivan).
* The FDA approves nevirapine (Viramune). This is the first anti-HIV drug in the class called non-nucleoside reverse transcriptase inhibitor (NNRTI).
* The Levine Committee calls for an overhaul of AIDS research at the National Institutes of Health (NIH), including a stronger role for the Office of AIDS Research (OAR) and increased support for vaccine-related and investigator-initiated research.
APPROVED
* The FDA approves the viral load test, a new diagnostic test that measures the level of HIV in the blood.
* International AIDS Vaccine Initiative (IAVI) forms to speed the search for an effective HIV vaccine.
* Brazil begins a national distribution of anti-retrovirals. They are the first developing country to do this.
* HIV is no longer the leading cause of death for all Americans ages 25 to 44, but remains the leading cause of death for African Americans in this age group. Also, the number of new AIDS cases diagnosed in the US declines for first time in the history of the epidemic, though incidence varies by sex, race, and ethnicity.
* The Joint United Nations Program on HIV/AIDS (UNAIDS) begins operations; it is established to advocate for global action on the epidemic, and to coordinate HIV/AIDS efforts across the UN system.
* "Hit hard, hit early" theory of HAART initiation is popular.
* Cover stories hailing AIDS breakthroughs and the "end" of the epidemic appear in The New York Times Magazine, The Wall Street Journal, and Newsweek.
* Using a mathematical model, David Ho and colleagues estimate that HIV infection might be eradicated in approximately 3 years using HAART. This model was later shown to be wrong.
* RITA! and other publications begin discussing the development of virus resistant to HIV drugs, an obvious issue overshadowing the hope provided by HAART.
* Researchers show KS is caused by a herpes virus (Human Herpesvirus-8 or HHV-8).
* Data presented at 3rd International Congress on Drug Therapy in HIV Infection focuses on positive effects of IL-2.
* Chemokine receptors CCR5 and CXCR4 are identified as the main co-receptors for HIV.
* AIDS researcher, David Ho, is TIME magazine's 1996 Man of the Year.
* Interest grows in HIV-positive patients whose disease does not progress to AIDS. Tony Fauci and colleagues publish an important paper in Science.
* The 11th International AIDS Conference ("One World, One Hope") in Vancouver, Canada highlights the effectiveness of HAART, creating period of optimism. At the conference, David Ho asserts that antiretroviral therapy might cure HIV infection.
* Abbott and Roche initiate studies that will examine the effect of combining ritonavir and saquinavir. Data are presented at 36th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC).
* 3rd National Conference on Human Retroviruses and Related Infections is held in Washington, DC.
1997
* The FDA approves the NNRTI delavirdine (Rescriptor).
* US Congress enacts FDA Modernization Act of 1997, codifying accelerated approval process, and allowing dissemination of information about off-label uses of drugs.
* The FDA approves the PI nelfinavir (Viracept).
* The FDA approves new formulation of saquinavir (Fortovase, a soft-gel formulation designed to improve absorption in the body).
* The FDA approves Combivir, a combination of zidovudine (Retrovir, AZT) and lamivudine (Epivir, 3TC).
* CDC reports annual AIDS deaths dropped in the US. In fact, AIDS-related deaths in the US decline by more than 40% compared to the prior year, largely because of HAART.
* Interest in treating HIV-positive patients during primary HIV infection grows as several cohorts are studied. A study in Science by Bruce Walker's group reports strong HIV-1-specific proliferative responses following treatment in acutely infected patients.
* President Clinton announces goal of finding an effective vaccine in 10 years and the creation of Dale and Betty Bumpers Vaccine Research Center.
* Case reports of "Crix Belly," "Buffalo Hump," and "Protease Paunch" are becoming widespread--early signs of what would generally become known as the "lipodystrophy" syndrome, a complex cluster of metabolic and morphologic changes seen in HIV-positive patients, usually while taking HAART.
* Studies in Science and other journals report that HAART fails to clear all virus from the body, even when therapy is started soon alter infection.
1997
* The Oncologic Drugs Advisory Committee (ODAC) recommends FDA approval of paclitaxel (Taxol) as second-line treatment for KS.
* ACTG 320 shows "induction/maintenance" (strategy of induction with triple-drug therapy and later maintenance with dual NRTI therapy to control infection) does not work.
* Drug resistance continues to be a hot topic for clinicians and researchers alike, as the "treatment-experienced" patient population becomes better characterized through "treatment failure."
* RITA! and other publications begin exploring new genotypic and phenotypic assays in development as potential ways to manage HIV drug resistance. However, many clinicians are not really sure what to do with this new information or how to interpret it, an issue that still rings true today.
* The 1st National AIDS Malignancy Conference is held at the NIH in Bethesda.
* The renamed 4th Conference on Retroviruses and Opportunistic Infections (CROI) is held in Washington, DC.
* 37th ICAAC highlights:
--"Hit hard, hit early" paradigm questioned as best management strategy
--Emerging data on HIV/HCV co-infection
--Study of Videx (didanosine, ddI) and hydroxyurea shows decreased viral load in patients with HIV
--Dual PI treatment strategy (including ritonavir as one PI) discussed by John Mellors: a prelude to the future role of boosted PIs as a virtual standard of care
1998
* Treatment Action Campaign (TAC) forms in South Africa as a grassroots movement pushing tar access to treatment.
* African Americans account for 49% of AIDS deaths in the US. Mortality for African Americans is almost 10 times that of whites and 3 times that of Hispanics.
* The FDA approves the NNRTI efavirenz (Sustiva).
* The FDA approves the NRTI abacavir (Ziagen).
* African-American leaders declare an HIV/AIDS "state of emergency" in US.
* The US Supreme Court in Bragdon v. Abbot rules that the Americans with Disabilities Act covers those in earlier stages of HIV disease, not just AIDS.
* The CFA, together with the Bering Community Service Foundation and Treatment Action Group in New York, hosts the first Houston Treatment Advocacy Forum.
* Despite optimism about HAART and apparent decreased incidence of and mortality from opportunistic infections (OIs), several reports indicate growing signs of treatment failure and side effects.
* AIDS groups protest DuPont's pricing of efavirenz (Sustiva) by dumping empty pill bottles from a black coffin. Members of ACT-UP storm DuPont's New York offices demanding the company cut the price of efavirenz. The CFA takes a controversial public stance supporting DuPont's pricing of efavirenz in the name of HIV research and development.
* Roy Gulick and a multicenter team report the longest patient follow-up to date showing that the simultaneous initiation of indinavir (Crixivan), zidovudine (Retrovir, AZT), and lamivudine (Epivir, 3TC) therapy is superior to sequential initiation (JAMA).
* April 24, 1998: Because of the rapid advances in the field of HIV research (primarily the development of antiretroviral therapy and measurement of viral load) there is a paradigm shift in the way HIV is treated, in contrast to previous guidelines, which focused on preventing and treating OIs. The US Department of Health and Human Services (DHHS) and CDC issue Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents and the Report of the NIH Panel to Define Principles of Therapy of HIV Infection.
* June 17, 1998: US guidelines recommend that treatment be offered to patients with acute syndrome, those within 6 months of seroconversion, and patients with symptomatic disease. For asymptomatic HIV-positive
patients, the guidelines recommend treating individuals with fewer than 500 CD4 T cells or plasma HIV RNA (viral load) levels over 20,000 copies/mL (using the RT-PCR assay).
* Bruce Walker's group at Massachusetts General Hospital takes one HIV-positive patient off HAART to see if his immune system will be able to actively control his infection. The volunteer had been receiving HIV medications for about a year and a half and began treatment almost immediately after infection. This case study introduces the theory that initiating HAART during primary (acute) HIV infection (or PHI) can alter the immune system's ability to control HIV in the long term. This represents one of the earliest attempts to study "structured treatment interruptions" during PHI.
* Several studies from around the world show induction/maintenance strategy is not feasible using currently available antiretroviral agents.
* A paper in The New England Journal of Medicine reports that zidovudine (Retrovir) reduces rates of perinatal transmission, even when used as an abbreviated regimen that is begun intrapartum or in the first 48 hours of life. Previous recommendations included a 3-part regimen over many weeks, which is not always possible.
* Reports of cardiovascular problems in patients being treated with PIs begin to appear in the literature.
* Several researchers report body changes that appear to be related to new combination drug therapies, particularly those that include a PI. Letters to the editor of the The Lancet discuss abnormal fat distribution in HIV-positive patients on PI therapy. A report in AIDS by Andrew Carr and colleagues describes the physical and biochemical changes occurring in HIV-positive patients taking PIs, defining lipodystrophy syndrome for the first time.
* VaxGen Inc. wins federal support tot a 3-year AIDS vaccine study (AIDS/Vax) involving 7,500 healthy human volunteers. This is the first large-scale human trial and first phase III trial to determine the efficacy of the gp 120-based vaccine.
* The 5th CROI is held in Chicago. The strategy of "bit hard, hit early" continues to be questioned. The opening address discusses lymph nodes as main reservoirs of HIV after HAART.
* Interest in drug resistance increases as treatment failures grow and transmission of resistant virus is documented in the Journal of Virology, The New England Journal of Medicine, and other journals.
* The 12th International AIDS Conference ("Bridging the Gap") is held in Geneva, Switzerland.
1999
* Multicenter AIDS Cohort Study (MACS) reaches 15-year mark.
* Studies begin to see increased rash in women versus men using nevirapine (Viramune).
* The FDA approves the PI amprenavir (Agenerase).
* The FDA approves a supplement to the Amplicor HIV-1 Monitor Test, extending the lower limit of viral load quantification from 400 to 50 copies/mL.
* ViroLogic, Inc. announces the commercial availability of PhenoSense, the company's rapid phenotypic HIV drug resistance assay.
* Multiple groups report continued viral replication even in patients with "undetectable" viral loads, suggesting that viral reservoirs persist even in the presence of suppressive therapy. Eradication of HIV with current medications is deemed unlikely.
* Studies suggest that many newly infected HIV-positive individuals are carrying forms of the virus that are already resistant to some antiretrovirals (20% to 30%).
* AIDS becomes the world's deadliest infectious disease in the last year, overtaking tuberculosis and moving up to 4th place among all causes of death worldwide, according to WHO.
* Bristol-Myers Squibb sends a warning letter to healthcare providers regarding pancreatitis with the NRTI didanosine (Videx, ddI).
* In an article published in The Lancet, doctors in Scotland warn against a possible interaction between sildenafil (Viagra) and PIs.
* Studies continue to report increased incidence of heart attack in patients taking HAART.
* Debate continues regarding the use of therapeutic drug monitoring, which has since been adopted as standard of care in some European countries, but not in the US.
* Resistance testing (genotypic and phenotypic) is evaluated as a potential tool for clinical management of patients.
* Researchers, particularly in the United Kingdom and Europe, emphasize that there may not be a need to initiate treatment until CD4 counts approach 200.
* Reports surface of lipodystrophy in HIV-positive patients not taking PIs.
* The Wall Street Journal and The New Fork Times Magazine publish articles on the "Berlin Patient." As reported at the 6th CROI, this patient showed no evidence of virologic rebound during the 551 days following permanent discontinuation of HIV therapy. HIV was still detected in the patient's lymph nodes and in resting CD4 T cells but at a low frequency.
* Interest and research into structured treatment interruptions (also known as strategic treatment interruptions or STIs) in the non-PHI setting explodes as the theory of "autovaccination" becomes very popular. Such therapy interruptions, researchers speculate, might act as a vaccine.
* The 6th CROI is held in Chicago. Several groups investigate the addition of IL-2 to a HAART regimen. Researchers from the National Institutes of Allergy and Infectious Diseases (NIAID) report that patients receiving IL-2 and HAART have much lower levels of resting CD4 T cells carrying replication-competent HIV than patients receiving HAART alone. However, a study in The Lancet reports that HIV activity continues in patients with successful viral suppression whether or not they received IL-2.
* A monumental study (HIVNET 012) is conducted in Uganda with results published in The Lancet. The study finds that a single dose of nevirapine (Viramune) taken orally by the mother while in labor, followed by a dose for the baby 3 days after birth, reduces the HIV transmission rate by half compared to a similar short course of zidovudine (Retrovir, AZT). This is an important move to simplify regimens to reduce mother-to-child transmission in resource-poor settings. (In 2004, questions will arise about the ethics of this trial with regard to the development of drug resistance, deaths caused by adverse reactions, etc.)
* Hope for STIs hinges on the observations from scientists at Massachusetts General Hospital that while HIV comes back each time the drugs are halted, there are signs the patients' immune systems are fighting to control the virus, with growing success.
1999
* The International Perinatal HIV Group publishes a meta-analysis of 15 prospective cohort studies in The New England Journal of Medicine showing that elective Caesarean section cuts the risk of HIV transmission in half
* 1st International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV is held in San Diego. At the workshop, a theory gains attention that mitochondrial dysfunction may be a component of lipodystrophy syndrome, specifically mitochondrial toxicity caused by NRTIs.
* May 1999: Meeting titled The Challenges of Clinical Trial Design in Assessing the Effects of Anti-HIV Therapy in Heavily Pre-treated Patients is held in Toronto, Canada. (The CFA cosponsors a follow-up meeting, The Salvage Therapy Think Tank, in April 2004.)
* Two major studies investigate the effect of IL-2: ESPRIT (Evaluation of Subcutaneous Proleukin in a Randomized International Trial) and SILCAAT (Subcutaneous IL-2 in HIV-infected Patients with Low CD4 Counts under Active Antiretroviral Therapy). ESPRIT investigators will accrue 4,000 patients and randomize them 1:1 to antiviral therapy plus or minus IL-2 and follow them for at least 4 years. SILCAAT is a 4-year trial in which researchers will randomize 1400 subjects with CD4 counts between 50 cells and 300 cells to IL-2 plus antiviral therapy or antiviral therapy alone. Chiron Corporation is the maker of Proleukin brand IL-2 and the study sponsor for SILCAAT. The NIH is sponsoring ESPRIT.
2000
* The FDA approves Kaletra, a combination pill containing 2 PIs, lopinavir and ritonavir (Norvir), where a small dose of ritonavir is included to boost levels of lopinavir.
* The FDA approves Glaxo Wellcome's application to market Trizivir. Trizivir combines the NRTIs zidovudine (Retrovir), lamivudine (Epivir), and abacavir (Ziagen) into one tablet.
* President Clinton announces the Millennium Vaccine Initiative, creating incentives for the development and distribution of vaccines against HIV, tuberculosis, and malaria.
* US and UN Security Councils declare HIV/AIDS an international security threat.
* South African president, Thabo Mbeki, embraces the denialist theory that HIV does not cause AIDS, thereby hindering efforts to treat HIV-positive patients in his country.
* The FDA approves a film-coated nelfinavir (Viracept) tablet (for ease of administration).
* The FDA approves Bristol-Myers Squibb's enteric-coated didanosine (Videx EC) capsules.
* CDC reports that, among men who have sex with men in the US, African-American and Latino cases of HIV exceed those among whites.
* Kiyoshi Kuromiya, founder of Critical Path AIDS Project, dies on May 9, 2000.
* UNAIDS, WHO, and other global health groups announce joint initiative with 5 major pharmaceutical manufacturers to negotiate reduced prices tot AIDS drugs in developing countries.
* CDC statistics: The estimated number of AIDS cases diagnosed in 2000 in the US is 41,267. The estimated number of people living with AIDS in the US is 334,731. The estimated number of deaths of persons with AIDS in the US in 2000 is 17,741. Cumulative deaths from AIDS since the start of the epidemic number 459,518 people.
* The 7th CROI is held in San Francisco.
* Attention grows regarding co-infection with hepatitis C virus (HCV) as a major co-morbidity in HIV-positive patients.
* US Congress reauthorizes the Ryan White CARE Act for the second time.
* In May 2000, a panel of the International AIDS Society-USA (IAS-USA) endorses the use of drug resistance testing.
* September 2000: Bruce Walker's group publishes a paper in Nature about initiating HAART in patients with PHI. The researchers conclude that there may be a benefit to this strategy alter patients were able to maintain a degree of viral control (5 out of 8 subjects remained off therapy with viral loads of less than 500 copies/mL after a median 6.5 months of stopping HAART). Measures of virus-specific immune function in these patients were also increased.
* Warning is issued about potentially fatal hepatotoxicity seen with nevirapine (Viramune). Patients taking nevirapine should be monitored carefully during the first 12 weeks of nevirapine therapy.
* Interactions become apparent between PIs and certain statins, a class of drugs increasingly used to treat hyperlipidemia as a result of HAART. A degenerative muscle condition called rhabdomyolysis can result from this drug interaction.
* The first CFA Basic Science Workshop is held on October 12, 2000.
* Entry inhibitors gain attention as a potential new class of antiretrovirals.
* HAART and cancer: While incidence of KS dramatically decreases in the era of HAART, other cancers persist, illustrating new health challenges faced by people living with HIV in the HAART era.
--Incidence of KS and primary brain lymphoma in people living with HIV/AIDS has declined, but trends are less certain for cervical, anal, and lung cancers as some research indicates the incidences of those cancers may have increased.
--According to a 2000 study conducted by the International Collaboration on HIV and Cancer, incidence of KS and non-Hodgkin's lymphoma (NHL) are lower in the era of HAART. Rates of other cancers seem unchanged.
* November 1, 2000: The New York Times reports that the Immune Response Corporation "tried to block the publication of a scientific paper that showed its HIV vaccine "Remune" was not effective (ie, did not have a dramatic effect on disease progression), and it has asked for damages of more than $7 million from the universities and researchers who published the findings." The disputed paper is published in the same day's issue of JAMA. Remune was originally developed by Dr. Jonas Salk more than 10 years earlier as a possible therapeutic vaccine.
* 13th International AIDS Conference ("Breaking the Silence") is held in Durban, South Africa. This is the first International AIDS Conference to be held in a developing nation and heightens awareness of the global pandemic.
Breaking the Silence
* A paper published by Daniel Kuritzkes and colleagues in the Journal of Acquired Immune Deficiency Syndromes contributes to the body of evidence showing that dual NRTI therapy, although able to suppress virus for some patients for a limited duration, is not as strong as triple combination therapy in terms of keeping resistance at bay for the longest duration. Most of the remaining interest in dual NRTIs as suppressive therapy for HIV has waned by this point in the HAART era.
2001
* AIDS organizations mark the 20th anniversary of the AIDS epidemic in the US in June.
* The FDA approves tenofovir (Viread). This drug is a nucleotide reverse transcriptase inhibitor, similar in action to the NRTI class of HIV drugs.
* February 5, 2001: US guidelines are updated and now recommend delaying therapy in asymptomatic HIV-positive patients until CD4 T cells are less than 350 or viral load is greater than 55,000 copies/mL (with RT-PCR).
* The FDA approves the first genetic test designed to look at virus mutations in an HIV-infected person. The test is called "Truegene."
* Generic drug manufacturers offer to produce discounted, generic forms of HIV/AIDS drugs; several major pharmaceutical manufacturers agree to offer further reduced drugs prices in developing countries.
* HIV/AIDS has the distinction of being a leading cause of human death worldwide.
* The World Trade Organization, meeting in Doha, Qatar, announces the "DOHA Agreement" to allow developing countries to buy or manufacture generic medications to meet public health crises, such as HIV/AIDS.
* AIDS Treatment Activists Coalition (ATAC) is founded in August 2001 at a meeting in Houston hosted by The CFA (atac-usa.org).
* The CFA's patient newsletter HIV Treatment ALERTS! debuts.
* Study team led by Steve Deeks publishes research in The New England Journal of Medicine showing that drug-resistant virus may not be as fit (able to reproduce itself, kill T cells, etc.) as wild-type virus. This is some good news for treatment-experienced patients, and consensus grows that staying on failing regimens (when no new treatment options are available) is better than stopping medications all together.
* SMART study (Strategies for the Management of Anti-Retroviral Therapy) is launched on October 15, 2001. The study will follow 6,000 HIV-infected subjects for as long as 8 years and compares treatment strategies of continuous viral suppression versus intermittent therapy guided by CD4 counts.
* With an intriguing twist on STIs, Tony Fauci and colleagues produce data on a potential "7 day on, 7 day off" STI regimen.
2001
* With the strength and durability of a Kaletra-based regimen now established, the idea of boosting other protease inhibitors with ritonavir (Norvir) gains popularity.
* The 8th CROI, held in Chicago, is characterized by prolific studies on STIs. Disorders of bone metabolism are also discussed.
* The 1st International AIDS Society Conference ("Conference on HIV Pathogenesis and Treatment") is held in Buenos Aires, Argentina. The conference strategy is to alternate with the International AIDS Conference.
* Ignoring recommendations from the Surgeon General, the Bush Administration mandates abstinence-only HIV prevention programs and targets programs that do otherwise for audits by the US Government.
* Pfizer division Agouron Pharmaceuticals swiftly ends its development collaboration on Immune Response Corporation's Remune. The Immune Response Corporation eventually goes overseas to conduct further trials in larger populations.
2002
* UNAIDS reports that as of December 2002, women comprise about half of all people living with HIV/AIDS worldwide.
* The FDA approves Zerit XR, a new once-daily version of Zerit (stavudine), which never comes to market.
* The FDA approves OraQuick Rapid HIV-1 Antibody Test, the first rapid test to use a finger prick.
* The FDA approves a new 600-mg version of efavirenz (Sustiva) to reduce pill burden.
* US National Intelligence Council releases report on the "Next Wave" of the epidemic, focused on India, China, Russia, Nigeria, and Ethiopia.
* HIV is the leading cause of death worldwide, among those aged 15 to 59.
* Bristol-Myers Squibb issues a warning regarding lactic acidosis in patients taking stavudine (Zerit, d4T).
* May 27, 2002: Linda Grinberg, head of the Foundation for AIDS and Immune Research, dies.
* On December 5, 2002, The CFA hosts the 2nd Basic Science Workshop on HIV. The theme was novel therapeutic interventions.
* Since December 2002, several reports have described kidney problems in patients taking tenofovir (Viread).
* The AIDSinfo website (aidsinfo.nih.gov) is launched by the US DHHS for HIV/AIDS clinical trials and treatment information. The new site replaces the AIDS Clinical Trials Information Service (ACTIS, established in May 1989) and the HIV/AIDS Treatment Information Service (ATIS, established in October 1994).
* Research in the Journal of Acquired Immune Deficiency Syndromes documents the changing landscape of causes of HIV mortality. While decreases are observed in deaths from CMV, wasting, and HIV-associated dementia, increases are reported in deaths from sepsis, and diseases of the liver, kidney, and heart.
* Pharmacogenomics, a new frontier in research, enters the HIV arena. Research begins to show that genetic differences influence such outcomes as efavirenz (Sustiva) effectiveness and abacavir (Ziagen) hypersensitivity.
* ADAP under fire: 13 state AIDS Drug Assistance Programs (ADAPs) are forced to limit access to life-saving HIV medications for uninsured and underinsured Americans due to inadequate funding. An era of treatment "haves" and "have nots" in the US is ushered in.
* Chiron Corporation announces it is terminating the SILCAAT trial because of business reasons. In the months following, the HIV research community and activists scramble to find a solution by sharing resources with ESPRIT and merging the two studies to ensure continuation of the IL-2 research.
* June 2002: Save ADAP Committee is founded (part of ATAC).
* Studies presented at the 14th International AIDS Conference ("Knowledge and Commitment for Action") in Barcelona, Spain continue to suggest that treatment during acute HIV infection (before testing positive for antibodies) may preserve the immune system.
* Some 9th CROI (Seattle) highlights:
--Several adjunct therapies (including rosiglitazone) aimed at treating HIV lipodystrophy show little effect.
--STIs may be dangerous in people with very low T cell counts (below 200 and especially below 50).
--An estimated 850,000 to 950,000 people in the US are living with HIV, including 180,000 to 280,000 who do not know they are infected.
2003
* July 14: The Guidelines for the Use of Antiretroviral Agents in HIV Injected Adults and Adolescents, published by the US DHHS, is updated. The most striking change is the removal of a menu-style list of HIV medications from which a doctor could choose a main "anchor" drug (a PI or NNRTI) from one list and two "background" drugs (usually NRTIs) from another list for treating a patient. This is replaced with the actual recommendation of preferred regimens. The two preferred regimens are either:
1. Sustiva + Epivir + (Zerit or Viread or Retrovir), or
2. Kaletra + Epivir + (Zerit or Retrovir)
* The FDA approves the first entry inhibitor enfuvirtide Fuzeon, T20).
* The FDA approves the P1 fosamprenavir (Lexiva). Fosamprenavir is a prodrug of amprenavir (Agenerase), and is converted into amprenavir in the body. The new formulation is easier to take because it requires much fewer pills.
* The FDA approves the PI atazanavir (Reyataz).
* The FDA approves the NRTI emtricitabine (Emtriva).
* CDC statistics:
* The estimated number of AIDS cases diagnosed in 2003 in the US is 43,171.
* The cumulative number of AIDS diagnoses through 2003 is 929,985.
* The estimated number of people living with AIDS in the US is 405,926.
* The estimated annual number of deaths of persons with AIDS in the US is 18,017.
* The cumulative number of deaths of persons with AIDS through 2003 in the US is estimated at 524,060.
* The William J. Clinton Presidential Foundation secures price reductions for HIV/AIDS drugs from generic manufacturers to benefit developing nations.
* At the end of 2003, only 35 states are conducting state-wide HIV surveillance using the same confidential name-based methods as used for AIDS surveillance. Because national HIV surveillance does not yet exist, only estimated numbers of HIV cases are available. At the end of 2003, there are an estimated 761,790 people diagnosed and living with HIV or AIDS. There are an estimated 366,000 people diagnosed and living with HIV (not AIDS).
* UNAIDS Report:
* In 2003, almost 5 million people became newly infected with HIV, the greatest number in any one year since the beginning of the epidemic.
* At the global level, the number of people living with HIV continues to grow--from 35 million in 2001 to 38 million in 2003.
* In 2003, almost 3 million people died of AIDS. More than 20 million people have died since the first cases of AIDS were identified in 1981.
* L. Joel Martinez, a founder of The CFA, dies on November 12, 2003.
* The South African Government announces a new antiretroviral treatment program.
* Interim analysis of study ACTG 5095 indicates that a triple-nuke regimen, in particular Trizivir, is not as effective as Sustiva-based regimens. Trizivir loses ground as a complete 3-in-1 regimen.
* Community HIV/AIDS Mobilization Project (CHAMP) is founded (champnetwork.org).
* "3 by 5" Initiative announced by WHO to bring HIV treatment to 3 million people by 2005.
* Carlton H. Hogan dies on November 18, 2003.
* The 10th CROI is held in Boston.
* Bernard Hirschel declares the autovaccination hypothesis to be dead (RITA! Fall 2003).
* The 2nd International AIDS Society Conference "Conference on HIV Pathogenesis and Treatment" is held in Paris, France.
* President Bush announces PEPFAR, the President's Emergency Plan for AIDS Relief, during the State of the Union Address. PEPFAR is a 5-year, $15-billion initiative to address HIV/AIDS, tuberculosis, and malaria primarily in hard-hit countries.
* December 2, 2003 (the day alter World AIDS Day): Abbott Laboratories raises the price of Norvir by more than 400% and ensures that its own boosted product, Kaletra, becomes the cheapest boosted PI on the market.
* Study CPCRA 064 confirms what many suspected--that STIs are dangerous in patients who have multi-drug resistant HIV. Although some smaller studies show more promising results, this is the beginning of work showing that STIs may not be beneficial, depending on the patient population.
2004
* Research presented by Steve Deeks (presented at CROI) and studies like PLATO (published in The Lancet) continue to show that patients with multi-drug-resistant (MDR) virus carl achieve a survival benefit (for example, less CD4 decline and stable viral load) by staying on a failing drug regimen (for instance with NRTIs or PIs). The strategic use of drug resistance to minimize viral replication capacity/fitness and pathogenicity becomes a realistic approach to help patients with few treatment options stay alive longer.
* The FDA approves Truvada, a fixed-dose, once-daily, co-formulation of the NRTIs emtricitabine (Emtriva, FTC) and tenofovir (Viread).
* The FDA approves Sculptra to treat facial lipoatrophy.
* The 11th CROI is held in San Francisco.
* The FDA approves Epzicom, a fixed-dose, once-daily, co-formulation of the NRTIs lamivudine (Epivir, 3TC) and abacavir (Ziagen).
* The FDA approves a generic version of didanosine (ddI) delayed release capsules (200 mg, 250 mg, and 400 mg). This is the first generic version of an HIV medication to be approved in the US.
* US DHHS announces expedited review process by the FDA for fixed-dose combination and co-packaged products to be used by the US in purchasing medications under PEPFAR.
* Evidence suggests that the combination ofdidanosine (Videx) and tenofovir (Viread) is not optimal.
* The FDA approves the OraQuick Rapid HIV-1 Antibody Test fin use with oral fluid.
* The Global Fund to Fight AIDS, Tuberculosis, and Malaria holds first ever "Partnership Forum" in Bangkok, Thailand; 400 delegates participate.
* With several major phase II studies in progress or set to launch, hope for oral entry inhibitors is at an all-time high.
* The CFA, in collaboration with the Forum for Collaborative HIV Research, hosts the Salvage Therapy II Think Tank on April 16-17.
* Group of 100 ADAP advocates from 30 different states travel to Washington, DC in February to ask lawmakers to consider a $180 million emergency supplemental appropriation for ADAP.
* Thousands of patients nationwide are on ADAP waiting lists as resources to cover medications no longer meet the need. Many states close enrollment, limit access to antiretrovirals, or anticipate program restrictions in the coming months. President Bush autborizes an emergency $90 million for states with ADAP waiting lists--the equivalent of a band-aid for the problems being caused by severe shortfalls in ADAP funding.
* UNAIDS launches The Global Coalition on Women and AIDS to raise the visibility of the epidemic's impact on women and girls around the world.
* 2004 statistics from UNAIDS:
* The number of people estimated to be living with HIV/AIDS worldwide is 39.4 million.
* The number of people newly infected with HIV worldwide is an estimated 4.9 million.
* The annual number of deaths by AIDS woldwide is an estimated 3.1 million.
* October 29, 2004: guidelines now recommend for HIV-positive patients who have never taken HIV medications before, have no symptoms, and whose T cell counts are over 350 should consider treatment when their HIV viral load reaches 100,000. The guidelines previously recommended considering treatment when viral load reached 55,000.
* Charles Clifton, the Executive Director of Test Positive Aware Network (TPAN) in Chicago and Editor of Positively Aware, dies on August 15, 2004.
* Keith Cylar, long time AIDS activist and co-founder and co-president of Housing Works, Inc., dies on April 5, 2004.
* New website is launched for AIDS Treatment News, a respected HIV newsletter since 1986 (aidsnews.org).
* 15th International AIDS Conference ("Access for All") is held in Bangkok, Thailand. This is the first International AIDS Conference to be held in Southeast Asia.
* In December, Bristol-Myers Squibb and Gilead Sciences announce that they will work together to develop a fixed-dose combination of efavirenz (Sustiva, an NNRTI) and the NRTIs emtricitabine (Emtriva) and tenofovir (Viread)--all in one pill, once a day.
* An activist campaign is launched against Abbott in response to its 400% price increase for the PI ritonavir (Norvir), commonly used to boost levels of other PIs to improve potency and durability of Pl-containing HAART. Notably, HIV-treating physicians join the campaign by barring Abbott representatives from their offices and refusing to consult with the company. A new group, Organized HIV Healthcare Providers (OHHP), is formed. For more information, visit atac.infovine.com/default/Abbottpricehike.asp or just Google "Abbott Norvir Price."
* The controversial HIVNET 012 nevirapine (Viramune) study is endorsed by the WHO, UNAIDS, and other international organizations. NIH stands by the results, including the safety and effectiveness of the single-dose nevirapine regimen for preventing transmission to infants during birth.
* New precautions are added to the labeling for the NNRTI nevirapine (Viramune). It should not be used in women with T cell counts greater than 250 or men with T cell counts greater than 400 because of the higher risk of developing liver damage.
2005 (through June)
* A new 500-mg tablet of the PI, Invirase, is now available to be used with ritonavir (Norvir) boosting.
* In February 2005, the FDA approves the combination of Pegasys (peg-Interteron) and Copegus (ribavirin) for the treatment of HCV in HIV-positive patients. This treatment is the first one approved for patients co-infected with HIV and HCV.
* The FDA approves the PI tipranavir (Aptivus) to be taken in combination with ritonavir (Norvir) for patients who have virus that is resistant to other PIs.
* On January 21, 2005, the CDC publishes new guidelines for the use of HIV medications to prevent HIV infection. Previously, post-exposure prophylaxis was only recommended for healthcare workers exposed to HIV. Now, HIV medications can be given to prevent infection after exposure to HIV through sexual intercourse, rape, injection drug use, or by accident.
* Warnings about using the PI Crixivan in HIV-positive pregnant women are published.
* At a historic and unprecedented joint press conference, the WHO, UNAIDS, the US Government, and the Global Fund to Fight AIDS, Tuberculosis, and Malaria announce results of joint efforts to increase the availability of antiretroviral drugs in developing countries. An estimated 700,000 people had been reached by the end of 2004.
* The CFA commemorates its 10th year and changes its name to "The Center for AIDS Information & Advocacy."
* March 2005: the SMART Study, continuing international expansion, reaches a halfway point and enrolls its 3000th patient. More than 200 US and international sites are enrolling patients.
* January 2005: Serono announces completion of patient enrollment in a phase III trial of its human growth hormone product Serostim for the treatment of HIV-associated lipohypertrophy. The primary goal of this trial is to assess whether Serostim induction therapy can reduce the abnormal accumulation of visceral adipose tissue and fat maldistribution seen in people with HIV/AIDS, and whether low-dose maintenance therapy prevents the abnormalities from returning during a continued course of therapy. More than 300 patients were enrolled in the study in 6 months.
* The 12th CROI is held in Boston.
* The 3rd International AIDS Society Conference "Conference on HIV Pathogenesis and Treatment" is to be held in Rio de Janeiro, Brazil.
* Study at 12th CROI by Walensky and colleagues reports 200 million years of life saved by HAART. Media quotes lead investigator as saying that HAART "can lengthen the life span of persons with AIDS by nearly 15 years."
* In May, more than 3,500 people living with HIV/AIDS and their loved ones from across the nation converge on Washington, marking the public step-off of the Campaign, to End AIDS (C2EA). Protesters marched down Pennsylvania Avenue, then lined up 8,500 pairs of donated shoes in the street directly in front of the White House to symbolize the number of people worldwide who die of AIDS daily. The march was timed with the launch of a new website, endAIDSnow.org, which features a 21-point plan to halt the epidemic worldwide.
COPYRIGHT 2005 The Center for AIDS: Hope & Remembrance Project
COPYRIGHT 2005 Gale Group