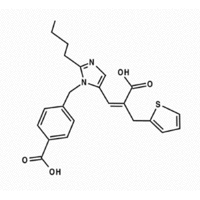
Biovail Corporation (NYSE:BVF)(TSX:BVF), Toronto, has announced that the United States Food and Drug Administration (FDA) has approved Teveten(R) HCT for the treatment of hypertension (high blood pressure). Teveten(R) HCT is a combination of the angiotensin II receptor blocker (ARB), eprosartan mesylate, and the diuretic, hydrochlorothiazide. Biovail will begin shipping Teveten(R) HCT to the trade and initiate promotional activities for the two dosage strengths of this combination ARB/diuretic product in early March 2003.
"Most patients with high blood pressure, regardless of age, race, gender or severity of hypertension, respond to Teveten(R)," commented Eugene Melnyk, chairman and CEO of Biovail. "With the availability of Teveten(R) HCT in two dosing strengths, Biovail is offering physicians the flexibility to provide their patients with additional control."
Studies illustrate that approximately two thirds of hypertensive patients require the use of more than one therapeutic agent to bring their blood pressure to an acceptable target level. Teveten(R) HCT offers patients the convenience of a combination of a diuretic and an angiotensin receptor blocker (ARB) in one tablet. This product may be used alone or in conjunction with other antihypertension medications.
The ARB market segment is the fastest growing class of antihyperintensive medications in the United States. The total value of the ARB market is approximately $2.6 billion in the United States and growing in excess of 34% annually. The value of the ARB/hydrochlorothiazide market, to which Teveten(R) HCT belongs, is approximately $1 billion per year and growing at an annual rate of 44%.
Biovail acquired the United States marketing rights for Teveten(R) and Teveten(R) HCT from Solvay Pharmaceuticals in March of 2002. Under the terms of this agreement, Solvay manufactures and supplies both products to Biovail for U.S. distribution. The chemical compound, eprosartan mesylate, has patent protection until 2011.
The Prescribing Information for Teveten(R) HCT states that this product should not be taken during the second and third trimesters of pregnancy.
Biovail Corporation is an international full-service pharmaceutical company, engaged in the formulation, clinical testing, registration, manufacture, sale and promotion of pharmaceutical products utilizing advanced drug delivery technologies.
For further information, call 905-286-3000.
COPYRIGHT 2003 Worldwide Videotex
COPYRIGHT 2003 Gale Group