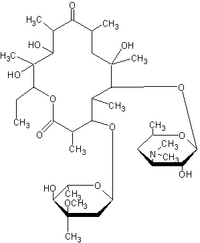
Erythromycin
Erythromycin is a macrolide antibiotic which has an antimicrobial spectrum similar to or slightly wider than that of penicillin, and is often used for people who have an allergy to penicillins. For respiratory tract infections, it has better coverage of atypical organisms, including mycoplasma. It is also used to treat outbreaks of chlamydia, syphilis, and gonorrhea. Structurally, this macrocyclic compound contains a 14-membered lactone ring with ten asymmetric centers and two sugars (L-cladinose and D-desoamine), making it a compound very difficult to produce via synthetic methods. more...
Erythromycin is produced from a strain of the actinomyces Saccaropolyspora erythraea, formerly known as Streptomyces erythraeus.
History
Abelardo Aguilar, a Filipino scientist, sent some soil samples to his employer Eli Lilly in 1949. Eli Lilly’s research team, led by J. M. McGuire, managed to isolate Erythromycin from the metabolic products of a strain of Streptomyces erythreus found in the samples. The product was subsequently launched in 1952 under the brand name Ilosone® (after the Philippine region of Iloilo where it was originally collected from). Erythromycin was formerly also called Ilotycin®. In 1981, Nobel laurate (1965 in chemistry) and Professor of Chemistry at Harvard University (Cambridge, MA) Robert B. Woodward and a large team of researchers reported the first stereocontrolled asymmetric chemical synthesis of Erythromycin A.
Available forms
Erythromycin is available in enteric-coated tablets, oral suspensions, opthalmic solutions, ointments, gels and injections.
Mechanism of action
Erythromycin prevents bacteria from growing, by interfering with their protein synthesis. Erythromycin binds to the subunit 50S of the bacterial ribosome, and thus inhibits the translation of peptides.
Pharmacokinetics
Erythromycin is easily inactivated by gastric acids, therefore all orally administered formulations are given as either enteric coated or as more stable salts or esters. Erythromycin is very rapidly absorbed, and diffused into most tissues and phagocytes. Due to the high concentration in phagocytes, erythromycin is actively transported to the site of infection, where during active phagocytosis, large concentrations of erythromycin are released.
Metabolism
Most of erythromycin is metabolised by demethylation in the liver. Its main elimination route is in the bile, and a small portion in the urine. Erythromycin's half-life is 1.5 hours.
Side-effects
Gastrointestinal intestinal disturbances such as diarrhea, nausea, abdominal pain and vomiting are fairly common so it tends not to be prescribed as a first-line drug. More serious side-effects, such as reversible deafness are rare. Allergic reactions, while uncommon, may occur, ranging from urticaria to anaphylaxis. Cholestatic jaundice, Stevens-Johnson syndrome and toxic epidermal necrolysis are some other rare side effects that may occur.
Erythromycin has been shown to increase the probability of pyloric stenosis in children whose mothers took the drug during the late stages of pregnancy or while nursing.
Contraindications
Earlier case reports on sudden death prompted a study on a large cohort that confirmed a link between erythromycin, ventricular tachycardia and sudden cardiac death in patients also taking drugs that prolong the metabolism of erythromycin (like verapamil or diltiazem) by interfering with CYP3A4 (Ray et al 2004). Hence, erythromycin should not be administered in patients using these drugs, or drugs that also prolong the QT time. Other examples include terfenadine (Seldane, Seldane-D), astemizole (Hismanal), cisapride (Propulsid, withdrawn in many countries for prolonging the QT time) and pimozide (Orap).
Read more at Wikipedia.org