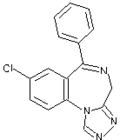
A dietary supplement was recalled after FDA determined that the product contained a prescription-strength sedative not listed on the label.
In March, FDA warned consumers not to buy or consume the supplement, known as Sleeping Buddha, which was marketed as an herbal alternative to prescription sedatives. The agency has never reviewed Sleeping Buddha or approved it for its promoted uses to treat insomnia and restlessness.
The prescription sedative ingredient, estazolam, is known to have serious side effects, including possible fetal damage if pregnant women take the drug. It also can pose a risk to consumers who drive, operate heavy machinery, or take other sedative drugs or drink alcohol while taking the drug.
Sleeping Buddha is marketed in capsule form as a product of China. Its distributor, Treasure Box Products Inc. of Burnaby, British Columbia, initiated the product recall in March, after FDA and Canadian testing identified the presence of estazolam.
FDA has received no reports of injuries from the product but advises consumers who have used Sleeping Buddha and have concerns to consult with their health-care providers.
COPYRIGHT 1998 U.S. Government Printing Office
COPYRIGHT 2004 Gale Group