Nicola, A. M.1; Andrade, R. V.2; Felipe. M. S. S.3
1,2,3 UnB - Department of Cell Biology
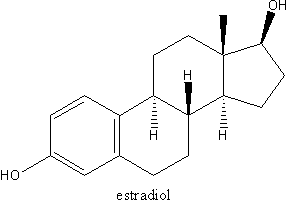
Introduction and objectives: Paracoccidioides brasiliensis is a dimorphic and human pathogenic fungus, responsible for paracoccidioidomycosis (PCM), the most prevalent systemic mycosis in Latin America. The morphologic transition from mycelium to yeast, which can be reproduced in vitro, is essential for the establishment of fungal infection and can be blocked both in viva and in vitro by the steroid hormone b-estradiol. This phenomenon has been postulated to be the cause of the early observed sexual bias in PCM. a disease which is found in an average of 20 men to one woman in spite of similar infection rates. In the early 1980's a group isolated biochemically cytoplasmic steroid receptors in several fungi, including P. brasiliensis and Candida albicans. The same group cloned and sequenced the C. albicans receptor, naming it EBP1. We used this sequence to search in the P. brasiliensis transcriptome database and found a cDNA named PbEBP, which we are now initially characterizing. Methods: Database search was done with the tBLASTn tool. The sequence was analyzed using free online bioinformatics tools. Total mycelium and yeast RNAs were obtained by extraction with the TRIZOL reagent, separated by denaturing electrophoresis, blotted onto nylon membranes and hybridized with a radioactively labeled PbEBP probe. Results: The tBLASTn with the C. albicans EBP1 sequence resulted in an alignment with e-value of 10^sup -56^ with a 1434 bp contig. The sequence presented an open reading frame encoding a putative protein with 395 aminoacid residues and predicted molecular weight of 43 KDa, which is very similar to C. albicans EBP1. The predicted protein sequence, when scare he with BLASTp agains GenBank, aligns with several FMN-binding oxidoreductases from bacteria, plants and fungi, including .V. cerevisiae Oye and C. albicans EBP1. Protein domain search using InterProScan (www.ebi.ac.uk/interpro) revealed a conserved NADH:flavin oxidoreductase motif. Prediction of its subcellular localization using PSORT II (psort.nibb.ac.jp) resulted in 69,6% chance of the protein being cytoplasmic. PbEBP has been shown to be differentially expressed in the yeast phase by electronic subtraction and microarray. We confirmed this expression pattern by Northern hybridization. Conclusion: The PbEBP cDNA encodes a protein which, after bioinformatics analyses, seems to be similar to the C. albicans estradiol binding protein. Although these results do not allow the conclusion that it is responsible for the physiological effects of estradiol in P. brasiliensis, we think that it is probably the longsought estradiol receptor. Additionally, we confirmed that the gene is differentially expressed in the yeast phase. Financial support: CNPq.
Copyright Instituto de Medicina Tropical de Sao Paulo Oct 2005
Provided by ProQuest Information and Learning Company. All rights Reserved