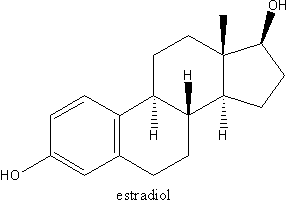
Androgens have direct central nervous effects that modulate endocrine factors associated with hot flashes. Combining androgen therapy with estrogens is an option for the relief of hot flashes in postmenopausal women.
**********
Menopause-related hot flashes are usually treated with a standardized dose of estrogen. This general approach to the most prevalent menopausal symptom is predicated on the belief that all menopausal women will respond to a uniform dose of estrogen, irrespective of the type or route of administration. Given the heterogeneity in clinical presentation and response to various doses of estrogen therapy, hot flashes may be considered a symptom of a multifactorial syndrome that involves estrogen deficiency as one of many other relevant vasoactive and endocrine factors. The role of androgens is central to this concept and the need for individualizing therapy.
CLINICAL DIFFERENTIATION
Excessive sweating that accompanies hot flashes is associated with labile skin conductance and a reduced sweating and shivering threshold, resulting in a narrowed thermoneutral zone (Figure). This physiological reaction has been attributed to the elevation of brain norepinephrine levels within the preoptic hypothalamus. (1) Patients with this symptom, which is responsive in some measure to estrogen treatment, benefit by the addition or substitution of other neuroendocrine agents that widen the thermoneutral zone.
[FIGURE OMITTED]
The thermoregulatory set-point in the hypothalamus may depend on the interaction and balance between estrogen and the hyperthermic (5-H[T.sub.2A]) and hypothermic (5-H[T.sub.1A]) receptors. Selective serotonin reuptake inhibitors reduce hot flashes by 50% to 60%. (2,3) This may be due to 5-H[T.sub.2A] blockade and 5-H[T.sub.1A] stimulation, which prevents hyperthermia and inhibits hypothermia, respectively.
ANDROGENS ARE PROHORMONES TO ESTROGEN
Adrenal androgens, DHEAS, DHEA, and androstenedione, contribute to 95% of the estrogenic activity in women and are responsible for more than 50% of the androgenic activity in women. Through genetically determined enzymes, these androgen prohormones are converted to androstenedione and then to testosterone and estrone. Testosterone is bioactive via aromatization to estradiol or by 5[alpha]-reductase activity to DHT, the most potent androgen.
SHBG: PHARMACOLOGICAL AND THERAPEUTIC IMPLICATIONS
Tissue uptake of sex steroids is governed by the binding to and rate of dissociation from its binding proteins, the rate of blood flow through the tissue, and the lipid solubility of the steroids. (7) SHBG, a hepatic-synthesized glycoprotein, retards the movement of testosterone (which is lipophilic) from blood into the CSF. (4)
The synthesis of SHBG is influenced by the type and route of estrogen treatment. After 3 months of treatment, conjugated equine estrogen, 0.625 mg/d orally, increases SHBG levels by 100% over baseline values. After 1 mg of 17[beta] estradiol orally and 50[micro]g of transdermal estrogen, SHBG levels increased 42% and 12%, respectively. (5) The addition of androgen to estrogen treatment modulates this response; 2.5 mg of methyltestosterone plus 1.25 mg of esterified estrogen daily decreased SHBG levels by more than 40%, and esterified estrogen alone at 1.25 mg/d increased plasma levels of SHBG by 95% after 3 months of treatment. (6)
Methyltestosterone has at least 2 other positive and relevant pharmacological actions that explain its ability to attenuate hot flashes. (6)
1. Combined esterified estrogen and methyltestosterone therapy significantly increases free testosterone levels after 3 months of therapy.
2. Short-term (6 days) and high-dose therapy with methyltestosterone (in men) resulted in a greater increase in CSF methyltestosterone levels than in plasma, a significant increase in CSF levels of 5-hydroxyindoleacetic acid (a measure of serotonin activity), and a significant decrease in the CSF norepinephrine metabolite, 3-methoxy-4-hydroxyphenylglycol. (7)
Clinical Message. Androgens are essential prohormones for estrogen synthesis. Methyltestosterone has some unique properties. It acts on both ERs and androgen receptors in a dose-dependent fashion; (8) it significantly lowers SHBG levels, thereby making more endogenous and prescribed estrogen and androgen bioavailable. (6) Additionally, methyltestosterone prevents suppression of adrenal androgen synthesis by estrogen treatment alone, thus allowing for enhanced peripheral androgen bioconversion to estrogen. (6) By lowering SHBG levels, methyltestosterone theoretically enhances the passage of estrogen and androgens across the BBB. (4) Furthermore, methyltestosterone crosses freely into the CSF and is associated with intracerebral biologic events relevant to the pathophysiology of hot flashes--serotonin production is increased and norepinephrine diminished. (7)
CLINICAL EXPERIENCE AND TRIALS
Experience in women who have undergone surgical menopause has shown a decrease in somatic symptoms (including hot flashes) after the use of intramuscular testosterone enanthate. (9) However, the beneficial effect may have been due in part to the bioconversion of testosterone to estrogen.
Hot flashes frequently persist despite adequate oral estrogen treatment. In one study, (10) 41% of menopausal women taking either conjugated equine estrogen, 1.25 mg, or estradiol valerate, 4 rag, daily reported persistence of hot flashes, and 35% had significant night sweats. Three months after subcutaneous implantation of testosterone (100 mg) and estradiol (40 mg), these values decreased to 19% and 13%, respectively. Thus, as is noted frequently in clinical practice, hot flashes are often more related to fluctuating estrogen concentrations and less often to absolute hormone values.
The addition of androgen therapy allows better symptom control with lower doses of estrogen treatment. In a placebo-controlled study, estrogen-androgen therapy (esterified estrogen, 0.625 mg, and methyltestosterone, 1.25 mg, daily) improved menopausal symptoms (hot flashes, night sweats, and vaginal dryness) to a significantly greater degree than did esterified estrogen, 0.625 mg/d, alone and placebo. (6) Higher doses of this combination (esterified estrogen, 1.25 mg; methyltestosterone, 2.5 mg) did not duplicate this androgen-enhancing effect but may be due to lower posttreatment levels of estradiol and estrone in the combined treatment group than in the group that received estrogen alone at 1.25 mg.
Clinical Message. Androgen therapy should be combined with estrogen therapy since estrogen up-regulates the androgen receptor, thus facilitating androgen expression; estrogen may also modulate the adverse effects of androgens. Persistent hot flashes in women receiving "adequate" estrogen therapy are often indicative of reduced bioavailable estrogen due to excess binding with SHBG. This can be confirmed with measured plasma FSH levels (as a surrogate of SHBG) in a "menopausal" range (>50 miU/L) and estradiol values in an appropriate "therapeutic" (premenopausal) range (>40-50 pg/mL). (10)
CLINICAL PRACTICE: CONSIDER THE INDIVIDUAL PATIENT
Clinicians evaluate and treat individual patients. An understanding of the physiology (and when relevant, the pathology) of the presenting condition and the pharmacology of relevant therapies allows individualization of care. In women with persistent hot flashes who are receiving adequate estrogen therapy, an androgen or androgenic progestin should be added to their treatment regimen (to decrease SHBG-estrogen binding) rather than an empirical increase in their estrogen dose. Methyltestosterone (which lowers triglyceride levels) would be the androgen of choice if the patient has concomitant hypertriglyceridemia; testosterone (which is lipid neutral) is preferred if the patient's high-density lipoprotein cholesterol level is low.
In brief, the skill and art of medical practice are to know when and how to apply validated scientific observations to the needs of the individual patient.
BBB = blood-brain barrier: CSF = cerebrospinal fluid: DHEAS = dehydroppiandrosterone sulfate: DHT = dihydrotestosterone: ER = estrogen receptor; FSH = follicle-stimulating hormone: 5-HT = 5-hydroxytryptamine: SHBG = sex hormone-binding globulin
Condensed and reprinted with permission from Mayo Clin Proc. 2004:79(suppl):S14-S18.
REFERENCES
(1.) Freedman RR. Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol. 1999;181:66-70.
(2.) Stearns V. Ullmer L, Lopez JF. Smith Y Isaacs C. Hayes D. Hot flushes. Lancet. 2002;360:1851-1861.
(3.) Loprinzi CI., Sloan JA. Perez EA. et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol, 2002;20:1578-1583.
(4.) Hobbs CJ, Jones RE, Plymate SR. The effect of sex hormone binding globulin (SHBG) on testosterone transport into the cerebrospinal fluid. J Steroid Biochem Mol Biol. 1992;42:629-635.
(5.) Nachtigall LE, Raju U. Banerjee S. Wan L. Levitz M. Serum estradiol-binding profiless in postmenopausal women undergoing three common estrogen replacement therapies: associations with sex hormone-binding globulin. estradiol, and estrone levels. Menopause, 2000:7:243-250.
(6.) Simon J, Klaiber E, Wiita B. Bowen A, Yang IIM. Differential effects of estrogen-androgen and estrogen-only therapy on vasomotor symptoms, gonadotropin sectretion, and endogenous androgen bioavailability in postmenopausal women. Menopause. 1999: 6:138-146.
(7.) Daly RC, Su TP, Schmidt PJ. Pickar D. Murphy DL. Rubinow DR. Cerebrospinal fluid and behavioral changes after methyltestosterone administration: preliminary findings. Arch Gen Psychiatry. 2001;58:172-177.
(8.) Papaconstantinou AD, Umbreit TH. Goering PL. Brown KM. Effects of 17[alpha]-methyltestosterone on uterine morphology and heat shock protein expression are mediated through estrogen and androgen receptors. J Steroid Biochem Mol Biol. 2002:82:305-314.
(9.) Sherwin BB, Gelfand MM. Differential symptom response to parenteral estrogen and/or androgen administration in the surgical menopause, Am J Obstet Gynecol. 1985;151:153-160.
(10.) Burger HG, Hailes J, Menelaus M. Nelson J. Hudson B. Balazs N. The management of persistent menopausal Symptoms with oestradiol-testosterime implants: clinical, lipid and hormonal results. Maturitas 1984:6:351-358.
Consultant, Adult Women's Health & Medicine, Boca Raton, Fla, and Washington, DC.
Dr Notelovitz has been on the Advisory Board for Solvay Pharmaceuticals, Inc, and serves oft the Speakers Bureau for Solvay Pharmaceuticals, Inc.
COPYRIGHT 2004 Dowden Health Media, Inc.
COPYRIGHT 2004 Gale Group