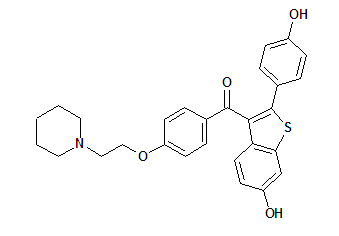
Eli Lilly and Co. has issued a warning to pharmacists regarding potential for errors in filling Evista prescriptions. Evista @ (raloxifene HCI) 60mg tablets, manufactured and sold by Eli Lilly and Co. and recently approved for the prevention of postmenopausal osteoporosis, is not the same as Vista (hydroxyzine HCI), formerly manufactured by Seatrace Pharmaceuticals. Generic hydroxyzine HCI should not be substituted for Evista; they are different products with different indications.
COPYRIGHT 1998 Reproduced with permission of the copyright holder. Further reproduction or distribution is prohibited without permission.
COPYRIGHT 2004 Gale Group