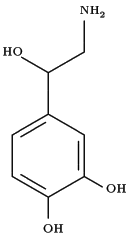
Background and objectives: Many clinicians believe that low-dose dopamine (LDD) [2 [micro]g/kg/min] increases renal blood flow (RBF) and medium-dose norepinephrine (MD-NE) [0.4 [micro]g/kg/min] decreases RBF. They also believe that MD-NE might induce mesenteric and/or coronary ischemia. In fact, the effects of these drugs on renal and vital organ blood flow are poorly understood. The aim of this study was to compare the effects of 6 h of IV LDD and MD-NE infusion on mammalian renal, coronary, mesenteric, and sagittal blood flow.
Design: Randomized, controlled, experimental animal study.
Setting: Animal laboratory of tertiary physiology institute.
Subjects: Seven Merino cross sheep were studied.
Measurements and results: We performed a staged insertion of transit-time flow probes around ascending aorta, sagittal sinus and circumflex coronary, superior mesenteric, and left renal arteries. We then randomized these animal with long-term embedded flow probes to either 6 h of placebo (saline solution) or drugs (MD-NE at 0.4 [micro]g/kg/min or LDD at 2 [micro]g/kg/min), and performed continuous measurement of systemic pressures, cardiac output (CO), and flow to vital organs. We also sampled blood and urine for the measurement of lactate, creatinine, and creatinine clearances at preset intervals.
Results: Compared to placebo, LDD did not affect systemic hemodynamies. However, it increased mean RBF by 20% (267.3 [+ or -] 87.6 mL/min vs 222.0 [+ or -] 74.4 mL/min, p = 0.028) without a detectable effect on other vital regional circulations. MD-NE, however, increased mean arterial pressure (101.0 [+ or -] 8.3 mL/min vs 84.2 [+ or -] 5.2 mL/min, p = 0.018) [mean [+ or -] SD] and CO (4.93 [+ or -] 1.45 L/min vs 3.81 [+ or -] 0.57 L/min, p = 0.028). It also increased coronary blood flow (36.0 [+ or -] 15.7 mL/min vs 23.0 [+ or -] 10.7 mL/min, p = 0.018) and RBF (286.5 [+ or -] 79.0 mL/min vs 222.0 [+ or -] 74.4 ML/min, p = 0.018). MD-NE had no detectable effect on mesenteric or sagittal sinus flow. LDD infusion increased urine output, but did not change creatinine clearance. MD-NE infusion increased urine output significantly more than LDD but not creatinine clearance.
Conclusions: Both LDD (2 [micro]g/kg/min) and MD-NE (0.4 [micro]g/kg/min) increased RBF and urine output. However, the effect of MD-NE was more pronounced. LDD did not affect other vital organ flows, but MD-NE increased coronary blood flow without any changes in mesenteric and sagittal sinus blood flow.
Key words: BP: cardiac output; coronary circulation; creatinine clearance; dopamine; mesenteric circulation; norepinephrine: renal circulation
Abbreviations: CO = cardiac output EMF = electromagnetic flow; HR = heart rate; LDD = low-dose dopamine; MAP = mean arterial pressure; MD-NE = medium-dose norepinephrine: RBF = renal blood flow; TPC =
total peripheral conductance
**********
Low-dose dopamine (LDD) is prescribed to increase renal blood flow (RBF) and attenuate or prevent renal ischemia (1-6) without decreasing other vital organ blood flow. However, the benefits of LDD have been challenged. (7,8) Norepinephrine has been used to induce acute renal failure in the animal and is, therefore, traditionally viewed as potentially injurious to the kidney (9-11) and other vital organs because of its vasoconstrictive properties. Much caution, therefore, is recommended in its use. (12,13) However, this view has been challenged. (14-19) Accordingly, it remains controversial whether LDD can best increase RBF, urine output, and creatinine clearance without compromisiug blood flow to other vital organs. We therefore conducted a randomized, crossover, animal experiment and measured RBF and vital organ blood flow (sagittal sinus, coronary artery, mesenteric artery) during the infusion of LDD (2 [micro]g/kg/min), medium-dose norepinephrine (MD-NE) [0.4 [micro]g/kg/min), or placebo.
MATERIALS AND METHODS
Animal Preparation
The local institutional Animal Ethics Committee approved this study. Seven Merino cross sheep weighing between 35 kg and 45 kg were procured for long-term instrumentation. The animals underwent four separate operative procedures for the placement of flow probes. The animals were induced with sodium thiopentone (15 mg/kg) for endotracheal tube placement (cuffed size 10). We maintained anesthesia by means of air, oxygen (initially set at a 0.5 fraction and then altered to maintain Pa[O.sub.2] at approximately 100 mm Hg), and isoflurane (0.5 to 2%). Ventilation was controlled to maintain PaC[O.sub.2] at approximately 40 mm Hg. Anesthetic management was the same for each operative procedure.
First, we performed an oophorectomy and fashioned bilateral carotid loops. We dissected 8 to 10 cm of mid-cervical carotid artery from the vagus, cervical sympathetic nerve, and minor blood vessels. The arteries were exteriotized below the carotid sinus baroreceptors, and the dissected arteries were enfolded in skin and sutured to form skin loops. We performed oophorectomy by ligation and removal of both intact ovaries via a midline laparotomy.
Secondly, we placed a sagittal sinus flow probe. The animal was placed in a stereotactic device (Howard Florey Institute; Melbourne, Australia). A longitudinal incision was made over the cranial vertex to expose the bregma, and craniotomy was then performed slightly anterior to the lambdoid suture to expose the dura mater and superior sagittal sinus. We made two longitudinal incisions on either side of the sinus for placement of the transit-time flow probe (4 mm) [Transonics Systems; Ithaca, NY]. We then covered the probe with silicone film and secured it in place with dental acrylic, which enveloped four stainless steel screws placed into the skull. The overlying skin was then closed. This technique has been previously described and validated as an accurate index of cerebral blood flow. (20) Flow probes were calibrated before insertion. By 1 week, the animal had fully recovered.
One week later, we performed a left-sided thoracotomy. We opened the pericardium to expose the heart and great vessels and placed a transit-time flow probe (3 mm) [Transonics Systems] around the circumflex artery, and an electromagnetic flow probe (20 mm) [In Vivo Metrics; Healdsburg, CA] around the ascending aorta.
Two weeks later, through a left-sided flank incision and retroperitoneal dissection, we exposed the superior mesenteric and left renal arteries and placed transit-time flow probes (6 mm and 4 mm, respectively) [Transonics Systems] around them. The animals were allowed to recover for 3 weeks. The use of long-term implanted transit-time flow probes has been previously validated. (21)
The transit-time flow probes were connected to a flowmeter (T20ICDS; Transonics Systems) via a four-channel sequential scanner (TM04; Transonics Systems). The electromagnetic flow (EMF) probes were activated by a Biotronex flowmeter (Biotronex; Kensington, MD). The output voltage of the EMF meter was reset to zero using an autozero circuit during a portion of diastole when blood flow in the ascending aorta is assumed to be zero. One month after implantation, the EMF probes were calibrated in vivo against thermodilution over a range of cardiac output (CO) values. Dobutamine was used to increase CO from approximately 4 to 9 L/min.
The day before experimentation, we inserted a carotid loop arterial Tygon catheter (inner diameter, 1.0 mm; outer diameter, 1.7 mm) [Cole-Parmers; Boronia, Australia] and two internal jugular venous polythene catheters (inner diameter, 1.2 mm; outer diameter, 1.7 mm) for the measurement of arterial and central venous pressures. The arterial and one venous cannula were connected to pressure transducers (TDXIII; Cube; Lakewood, CO) tied to the wool on the back of the sheep. A correction factor was added in the data collection program to compensate for the height of the transducer above the heart.
Pressure transducers for the measurement of arterial pressure and central venous pressure were tied to the back of the sheep and were calibrated against a mercury and water manometer, respectively. A value to compensate for the height of the transducers above the heart was added to the pressure readings. To measure the height above the heart, the heart level was taken as 64% of the distance from the back to the sternum, which is the junction of the left atrium with the left atrial appendage. The sheep were allowed 2 weeks to recover. The second venous catheter was used as an infusion line. A urinary catheter was inserted for urine flow measurements and sample analysis.
Analog signals (mean arterial pressure [MAP], central venous pressure, CO, change in flow over change in time, regional flows) were collected using a personal computer data acquisition system using custom software written at the Howard Florey Institute. Data were collected at 100 Hz for 10 s at 10-min intervals throughout the experimental protocol.
Protocol and Measurements
On the day of the experiment, we observed the animals for a 2-h preinfusion baseline period and than randomized them to either LDD, MD-NE, or placebo (vehicle) infusion.
The animals received either IV LDD (2 [micro]g/kg/min), MD-NE (0.4 [micro]g/kg/min), or the vehicle (as placebo) at the same infusion rate for 6 h. The doses were chosen on the basis of previous descriptions of LDD, (7,8) and the typical dose of norepinephrine reported for infusion in the critically ill. (12-14) The infusion was kept constant by means of peristaltic pump control (Perfusor; B. Braun; Melsungen, Germany). IV normal saline solution was administered to maintain central venous pressure constant (2 to 4 cm [H.sub.2]O). MAP, CO, heart rate [HR], sagittal sinus flow, coronary flow, mesenteric flow, and renal flow were measured continuously and recorded at 10-min intervals. Conductance was measured as the ratio between MAP and each regional flow. Urinary flow was measured continuously, and urine was sampled at 2-h intervals for analysis (Model 3CII Osmometer; Advanced lnstruments; Needham Heights, MA). Arterial blood samples were collected for analysis of serum urea, creatinine, and electrolytes (Beckman: Brea, CA) at 0 min, 30 min, 60 min, 180 min, and 360 min during the observation period. At the end of 6 h, the infusion was stopped. The animals were allowed to recover, The various catheters were removed. After 1 week, the animals were crossed over to one of the other arms of the study, After another week of rest, the animals were then crossed over to the remaining arm of the study.
Statististical Analysis
Data are presented as means with SDs. We used a Friedman test to detect any significant differences between placebo, LDD, and MD-NE infusion during the 6-h study period. We compared hemodynamics and RBFs between these three groups using the area under the curve method as described by Matthews et al. (22) If a difference was detected, subsequent direct comparison was performed using a Wilcoxon signed rank test, In this experiment, as the study period for each of the three treatments was the same, the mean value for each variable over the 6 h was used and presented as the statistical equivalent of the area under the curve. In addition, to better understand the determinants of regional organ flows, we conducted multivariate linear regression analyses including MAP, CO, totaI peripheral conductance (TPC), regional conductance, and using organ flow as the dependent variable. A p < 0.05 was considered statistically significant.
RESULTS
Systemic Hermodynamic Effects
Compared to placebo, LDD did not induce any changes in MAP (84.0 [+ or -] 6.1 mm Hg vs 84.2 [+ or -] 5.2 mm Hg, p = 0.61), CO (4.10 [+ or -] 0.82 L/min vs 3.81 [+ or -] 0.57 L/min, p = 0.091), or HR (64.1 [+ or -] 12.3 beats/min vs 63.8 [+ or -] 17.7 beats/min, p = 0.063) [Fig 1, top, a and b, and bottom, c]. However, compared to both placebo and LDD, MD-NE (0.4 [micro]g/kg/min) increased MAP (101.0 [+ or -] 8.3 mm Hg, p = 0.018 for both comparisons), CO (4.93 [+ or -] 1.45 L/min, p = 0.028 for both comparisons), and HR (76.2 [+ or -] 10.8 beats/min, p = 0.063). Neither drug affected calculated systemic conductance (Fig 1).
[FIGURE 1 OMITTED]
RBF and Urine Output
Compared to placebo, LDD increased RBF by 20% (267.3 [+ or -] 87.6 mL/min vs 222.0 [+ or -] 74.4 mL/min, p = 0.028) and renal conductance by 20% (3.19 [+ or -] 0.93 mL/min/mm Hg vs 2.65 [+ or -] 0.83 mL/ min/mm Hg, p = 0.028). MD-NE, however, increased RBF by 29% (286.5 [+ or -] 79.0 mL/min, P = 0.018) and renal conductance by only 8% (2.87 [+ or -] 0.80 mL/min/mm Hg, p = 0.018), suggesting that the increase in RBF was mostly secondary to increased renal perfusion pressure. There were no significant differences when LDD and MD-NE were compared. (Fig 2, top, a and b; Table 1). Compared to placebo, both LDD and MD-NE increased urine output (118.6 [+ or -] 35.7 mL/h for LDD vs 228 + 105 mL/h for MD-NE vs 49.0 [+ or -] 22.1 mL/h for placebo, p = 0.018 in both comparisons) [Fig 2, bottom, c]. On direct comparison, however, urine output was significantly higher with MD-NE than LDD (p = 0.028). Compared to placebo, LDD did not affect creatinine clearance (80.6 [+ or -] 11.8 mL/min vs 74.2 [+ or -] 11.8 mL/min, p = 0.091) (Fig 2, bottom, d). The change in creatinine clearance induced by MD-NE infusion just failed to achieve statistical significance (88.5 [+ or -] 12.2 mL/min, p = 0.067) [Fig 2, bottom, d] There were no significant differences in creatinine clearance when LDD and MD-NE were compared directly (p = 0.091).
[FIGURE 2 OMITTED]
Coronary Blood Flow
LDD did not change coronary flow or conductance compared to placebo. MD-NE infusion, however, increased coronary, flow by 57% (36.0 [+ or -] 15.7 mL/min vs 23.0 [+ or -] 10.7 mL/min, p = 0.018) with an associated 32% increase in coronary conductance (0.353 [+ or -] 0.141 mL/min/min Hg vs 0.268 + 0.121 mL/min/mm Hg, p = 0.028). On direct comparison, the difference between MD-NE and LDD in coronary blood flow was statistically significant (36.0 [+ or -] 15.7 mL/min vs 25.1 [+ or -] 9.7 mL/min, p = 0.028) [Fig 3, top, a and b; Table 1).
Mesenteric Flow
Compared to placebo, LDD infusion did not affect mesenteric blood flow (527.5 [+ or -] 179.0 mL/min vs 561.5 [+ or -] 165.2 mL/min, p = 0.61 ) or conductance (6.30 [+ or -] 1.99 mL/min/mm Hg vs 6.71 [+ or -] 1.91 mL/ min/mm Hg, p = 0.31) [Fig 4]. MD-NE infusion also did not change mesenteric blood flow or conductance (586.4 [+ or -] 267.9 mL/min [p = 0.74] and 5.76 [+ or -] 2.27 mL/min/mm Hg [p = 0.24], respectively) [Fig 4; Table 1]. Neither drug significantly affected lactate levels.
[FIGURE 4 OMITTED]
Sagittal Sinus Flow
Neither MD-NE nor LDD changed sagittal sinus blood flow (Table 1).
Multivariate Analysis
For all of the four vital organ flows, CO, TPC, and each regional conductance were found to be independent determinants of regional blood flows on multivariate linear regression analysis. MAP approximated significance only for RBF (Table 2).
DISCUSSION
We found that similarly to humans, in the sheep MD-NE increased MAP and CO, while LDD had no effect. LDD infusion, as expected, increased RBF, renal conductance, and urine output compared to placebo. Norepinephrine infusion at 0.4 [micro]g/kg/min (MD-NE), however, also increased RBF, renal conductance, and urine output. The increase in urine output was greater with MD-NE than LDD. MD-NE also increased coronary blood flow while LDD did not. Neither LDD nor MD-NE had major effects on mesenteric blood flow and conductance.
Systemic Effects
After a delay due to the time needed to transit through the central venous catheter, MD-NE infusion progressively increased MAP and CO, but LDD did not. These findings in systemic hemodynamics are in keeping with previous reports and are consistent with the known combined [alpha] and [beta]-adrenergic effects of norepinephrine and the lack of systemic hemodynamic effects of LDD. (1-7,11-19) These systemic effects of norepinephrine were probably, in part, responsible for the changes in organ blood flow. In this regard, they paralleled the increase in RBF and urine output (Figs 1-4). This observation highlights the influence increasing both CO and perfusion pressure on RBF. Multivariate regression analysis further confirmed the importance of systemic hemodynamic changes in determining regional blood flows.
Renal Effects,
LDD increased RBF, induced renal vasodilatation (as implied by increased conductance), and increased urine output (diuresis). These observations are consistent with numerous experimental observations.(23-27) However, MD-NE had similar effects. The mechanisms responsible for the increases in RBF, however, appeared different. The effect of LDD depended solely on changes in renal conductance, whereas the effect of MD-NE depended mostly on increased CO and renal perfusion pressure and only, in part, on changed renal conductance. These physiologic responses to norepinephrine are in keeping with previous experimental and human observations. (15-19,28-30) For example, Anderson et al (30) suggested that increased BP, CO, and an effect on renal sympathetic tone (local sympathetic system mediated renal vasodilation in response to increased BP) might be responsible for increased RBF and urine output during norepinephrine infusion.
Coronary Blood Flow
LDD did not affect coronary blood flow, while MD-NE infusion increased it by 57%. Previous animal experiments (31,32) have shown that dopamine does not affect coronary blood flow. Our results are consistent with these reports. Previous studies of the effect of norepinephrine on the coronary circulation or on blood flow in coronary artery gratis have demonstrated either a limited effect or a significant increase. (33,34) In our animals, MD-NE also increased coronary conductance. It is unclear whether this represents a direct vascular effect or a response of the coronary circulation to the [beta]-adrenergic receptor induced increase in CO mad myocardial oxygen demand.
Mesenteric Blood Flow
Neither LDD nor MD-NE affected mesenteric blood flow. LDD might be expected to affect mesenteric blood flow because mesenteric dopaminergic receptors have been documented in mammals. (35) However, their ability to respond to stimulation with vasodilatation remains untested. Our findings suggest that their stimulation does not induce significant mesenteric vascular changes. They are consistent with the findings of Pearson et al (36) who were unable to show mesenteric vasodilatation with LDD infusion in piglets. In fact, mesenteric dopaminergic stimulation may be dangerous during shock. Using a hemorrhagic shock model, some investigators have reported evidence of mesenteric ischemia during LDD infusion. (37) Previous experimental studies (38,39) of the effect of norepinephrine on the mesenteric circulation conducted in vasodilated septic animals found that norepinephrine neither increased nor decreased mesenteric blood flow.
Finally, we found no significant changes in sagittal sinus flow during the drug infusions. It is likely that, within the autoregulatory range, cerebral blood flow is not altered by the administration of these two vasoactive drugs.
Our study has some limitations. First, we used normal animals. Our results might not apply to animals in other physiologic or pathologic states. However, in our opinion, testing of drugs in normal animals should represent the necessary first experimental step before moving to tests in other pathophysiologic conditions. In addition, data in normal animals are neccssary to understand how the effect of a medication is altered by disease. Furthermore, if norepinephrine has no deleterious effect on renal, mesenteric, and coronary blood flow in the normal animal, it seems unlikely that it would have a deleterious effect in the setting of severe vasodilatation. We did not measure any dose-response relationship between drugs and regional flows mad cannot say what different doses of norepinephrine might do to regional flows. However, we selected the typical norepinephrine and LDD doses reported in the literature, (7,18,19) and studied their effects over a prolonged period. In particular, the 20% increment in MAP obtained with MD-NE is similar to the kind of effect considered desirable in critically ill patients.(17-19) Nonetheless, higher doses of norepinephrine might induce significantly different effects on vital organ blood flow. Some of our findings might be species specific. This is of course inherent to any animal study. The fact that similar observations (29,36) to ours have been made in dogs and piglets suggests that our observations might apply across different species. Finally, our observations in normal sheep suggest the need to extend our work to shock states, to dose-titration studies, and to include investigations of other frequently used vasoactive drugs such as vasopressin, epinephrine, and phosphodiesterase-III inhibitors.
In conclusion, although both LDD (2 [micro]g/kg/min) and MD-NE (0.4 [micro]g/kg/min) increased RBF and urine output, the effect of MD-NE was more pronounced. Neither drug had an effect on mesenteric blood flow. However, MD-NE increased coronary blood flow and CO. These observations suggest that the administration of MD-NE might be at least as physiologically rational as that of LDD.
REFERENCES
(1) Baldwin L, Henderson A, Hickman F, Effect of postoperative low-dose dopamine on renal function after elective major vascular surgery. Ann Intern Med 1994; 120:744-747
(2) Wee PM, Smith AJ, Rosman JB, et at. Effect of intravenous infusion of low-dose dopamine on renal function in normal individuals and in patients with renal disease. Am J Nephrol 1986; 6:42-46
(3) Chertow CM, Sayegh MH, Allgren RL, et al. Is the administration of dopamine associated with adverse or favorable outcomes in acute renal failure? Am J Med 1996; 101:49-53
(4) Kadieva VS, Friedman L, Margolius LP, et al. The effect of dopamine on graft function in patients undergoing renal transplantation. Anesth Analg 1993; 76:362-365
(5) Myles PS, Bucklaud MR, Schenk NJ, et al. Effect of "renaldose" dopamine on renal function following cardiac surgery. Anaesth Intensive Care 1993; 21:56-61
(6) Swygert TH, Roberts C, Valek TR, et al. Effect of intraoperative low-dose dopamine on renal function in liver transplant recipients. Anesthesiology 1991; 75:571-576
(7) Australian and New Zealand Intensive Care Society (ANZICS) clinical Trials Group. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomized trial. Lancet 2000; 356:2139-2143
(8) Kenum JA, Decker JM. Use of dopamine in acute renal failure: a meta-analysis. Crit Care Med 2001; 29:1526-1531
(9) Schafferhans K, Heidbreder E, Grimm D, et al. Norepinephrine-induced acute renal failure: beneficial effects of atrial natriuretic factor. Nephron 1986; 44:240-244
(10) Sinsteden TD, O'Neil TJ, Hill S, et al. The role of high-energy phosphate in norepinephrine-induced acute renal failure in the dog. Circ Res 1986; 59:93-104
(11) Schaer GL, Fink MP, Parfillo JE. Norepinephrine alone versus norepinephrine plus low-dose dopamine: enhanced renal blood flow with combination pressor therapy. Crit Care Med 1985; 13:492-496
(12) Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Practice parameters for hemodynamic support of sepsis in adult patients in sepsis? Crit Care Med 1999; 27:639-660
(13) Rudis MI, Basha MA, Zarowitz BJ. Is it time to reposition vasopressors and inotropes in sepsis? Crit Care Med 1996; 24:525-537
(14) Bellomo R, DiGiantomasso D. Noradrenaline and the kidney: friends or foes. Crit Care 2001; 5:294-298
(15) Martin C, Papazian L, Perrin G, et. al. Norepinephrine or dopamine for the treatment of hyperdynamic septic shock? Chest 1993; 103:1826-1831
(16) Martin C, Viviand X, Leone M, et. al. Effect of norepinephrine on the outcome of septic shock. Crit Care Med 2000; 28:2758-765
(17) Martin C, Eou B, Saux P, et. at. Renal effects of norepinephine used to treat septic shock patients. Crit Care Med 1990; 18:282-285
(18) Desjars P, Pinaud M, Bugnon D, et al. Norepinephrine therapy has no deleterious renal effects in human septic shock. Crit Care Med 1990; 18:1048-1049
(19) Redl-Wenzel EM, Armbruster C, Edelmann G, et at. The effects of norepinephrine on hemodynamics and renal function in severe septic shock states. Intensive Care Med 1993; 19:151-154
(20) Grant DA, Franzini C, wild J, et al. Continuous measurement of blood flow in the superior sagittal sinus of the lamb. Am J Physiol 1995; 269:R247-R279
(21) Bendarik AD, May CN. Evaluation of a transit-time system for the chronic measurement of blood flow in conscious sheep. J Appl Physiol 1995; 78:524-530
(22) Matthews LNS, Altman DG, Campbell MJ, et al. Analysis of serial measurements in medical research. BMJ 1990; 230-235
(23) Goldberg LI, McDonald RH Jr, Zimmerman AM. Sodium diuresis produced by dopamine in patients with congestive heart failure. N Engl J Med 1963; 269:1060-1064
(24) Hollenberg NK, Adams DF, Mendel] P, et al. Renal vascular responses to dopamine: hemodynamic and angiographic observations in normal man. Clin Sci Mol Med 1973; 45:733-742
(25) Bersten AD, Rutten AJ. Renovascular interaction of epinephine, dopamine, and intraperitoneal sepsis. Crit Care Med 1995; 23:537-544
(26) Fiser DH, Fewell JE, Hill DE, et al. Cardiovascular and renal effects of dopamine and dobutamine in healthy, conscious piglets. Crit Care Med 1988; 16:340-345
(27) Yura T, Yuasa S, Fukumaga M, et at. Role of Doppler ultrasound in the assessment of renal circulation: effects of dopamine and dobutamine on renal hemodynamics in humans. Nephron 1995; 71:168 175
(28) Zhang H, Small N, Cabral A, et al. Effects of norepinephrine on regional blood flow and oxygen extraction capabilities during endotoxic shock. Am J Respir Crit Care Med 1997; 155:1965-1971
(29) Bellomo R, Kellum JA, Wisniewski SR, et al. Effects of norepinephrine oil the renal vasculature in normal and endotoxemic dogs. Am J Respir Crit Care Med 1999; 159: 1186-1192
(30) Auderson WP, Korner PI, Selig SE. Mechanisms involved in the renal responses to intravenous and renal artery, infusions of noradrenaline in conscious dogs. J Physiol 1981; 321:21-30
(31) Bartelds B, Gratama JW, Meuzelaar KJ, et. al. Comparative effects of isoproterenol and dopamine on myocardial oxygen consumption, blood flow distribution and total body oxygen consumption in conscious lambs with and without an aortopulmonary left to right shunt. J Am Coll Cardiol 1998; 31:473-481
(32) Shi Y, Zalewski A, Bravette B, et al. Selective dopamine-1 receptor agonist augments regional myocardial blood flow: comparison of fenoldopam and dopamine. Am Heart J 1992; 124:418-423
(33) Mueller II, Ayres SM, Gregory JJ, et al. Hemodynamics, coronary blood flow, and myocardial metabolism in coronary shock; response to l-norepinephrine and isoproterenol. J Clin Invest 1970; 49:1885-1902
(34) DiNardo JA, Bert A, Sehartz MJ, et. al. Effects of vasoaetive drugs on flows through left internal mammary artery and saphenous vein grafts in man. J Thorac Cardiovasc Surg 1991; 102:730-735
(35) Missal C, Pizzi M, Memo M, et al. Postsynaptic D1 and D2 receptors are present in rabbit renal and mesenteric arteries. Neurosci Lett 1985; 61:207-211
(36) Pearson RJ, Barrington KJ, Jirsch DW, et al. Dopaminergic receptor-mediated effects in the mesenteric vasculature and renal vasculature of the chronically instrumented newborn piglet. Crit Care Med 1996; 24:1796-1712
(37) Segal J, Phang T, Walley K. Low-dose dopamine hastens onset of gut ischemia in a porcine model of hemorrhagic shock. J Appl Physiol 1992; 73:1159-1164
(38) Booke M, Hinder F, MeGuire R, et al. Nitric oxide synthase inhibition versus norepinephrine for the treatment of hyperdynamic sepsis in sheep. Crit Care Med 1996; 24:835-844
(39) Booke M, Hinder F, McGuire R, et al. Noradrenaline and N-monomethyl-L arginine (L-NMMA): effects on haemodynamics and regional blood flow in healthy and septic sheep. Clin Sci 2000; 98:19,3-200
* From the Deparment of Intensive Care (Drs. Di Giantomasso, Morimatsu, and Bellomo), Austin and Repatriation Medical Centre, Heidelberg; and Howard Florey Institute (Dr. May), Melbourne, VIC, Australia.
This study was supported by an institute grant (no. 983001) from the National Health & Medical Research Council of Australia, and by grants from the Intensive Care Foundation of the Australian and New Zealand Intensive Care Society, the Laerdal Foundation (Norway) and the Austin Hospital Anesthesia and Intensive Care Trust Fund.
Manuscript received March 21, 2003; revision accepted December 2, 2003.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (e-mail: permissions@chesnet.org).
Correspondence to: Rinaldo Bellomo, MD, Department of Intensive Care, Austin & Repatriation Medical Centre, Heidelberg, VIC 3084, Australia; e-mail: rinaldo.bellomo@armc.org.au
COPYRIGHT 2004 American College of Chest Physicians
COPYRIGHT 2004 Gale Group