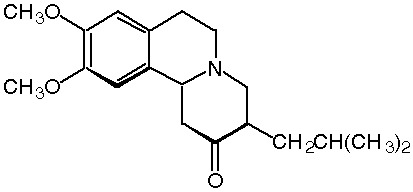
WASHINGTON -- Prestwick Pharmaceuticals, Inc. announced today that it has submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for review and approval to market tetrabenazine for the treatment of chorea associated with Huntington's disease. E[acute accent]Chorea is the most visible and common of the motor symptoms observed in patients with Huntington's disease. The condition is characterized by brief, irregular contractions that are not repetitive or rhythmic, but appear to flow from one body part to the next. There is currently no FDA-approved treatment for chorea associated with Huntington's disease in the U.S. E[acute accent]Tetrabenazine is a highly selective and reversible dopamine depletor that works by inhibiting vesicular monoamine transporter 2 (VMAT2). Tetrabenazine is approved for marketing outside the U.S. in eight countries and is under regulatory review for approval in several additional countries. Tetrabenazine is marketed in Europe and Australia by other sponsors under the brand name XENAZINE(R), and in Canada by Prestwick Pharmaceuticals Canada, Inc. under the brand name NITOMAN(R), for the treatment of hyperkinetic movement disorders, which includes Huntington's chorea. E[acute accent]Prestwick's NDA is supported, in large part, by Phase III clinical data that suggests tetrabenazine is significantly more effective than placebo in treating patients' chorea associated with Huntington's disease, with many patients demonstrating marked to good improvement. The most common side effects with tetrabenazine include sedation, Parkinsonism, depression and insomnia. The results of the Phase III clinical study were presented in October 2004 at the American Neurological Association 129th annual meeting in Toronto, Ontario. E[acute accent]The FDA has granted tetrabenazine orphan drug designation to treat a rare disease that affects less than 200,000 patients in the U.S. The FDA also has designated tetrabenazine a fast track product to facilitate the development and expedite its review as a new drug that's intended to treat a serious or life-threatening condition, and demonstrates its potential to address an unmet medical need.
E[acute accent]Prestwick Pharmaceuticals
E[acute accent]Prestwick Pharmaceuticals, Inc. is a product-focused specialty pharmaceutical company engaged in the development and commercialization of small molecule drugs with high commercial potential and relatively low development risk that target diseases of the central nervous system.
COPYRIGHT 2005 Business Wire
COPYRIGHT 2005 Gale Group