DUNDAS, Ontario -- Prestwick Pharmaceuticals Inc., a CNS specialty pharmaceutical company, announced today the planned launch of the NITOMAN(R) Access Hotline in Canada, a free telephone support service established to help patients suffering from hyperkinetic movement disorders to explore reimbursement options and obtain disease and treatment support in relation to NITOMAN therapy.
NITOMAN, a dopamine depletor that works by selectively blocking vesicular monoamine transporter 2 (VMAT2), is the only product approved in Canada for the treatment of a series of hyperkinetic movement disorders, including chorea associated with Huntington's Disease, tardive dyskinesia and Tourette's syndrome. Approximately 50,000 Canadians suffer from these disorders, which are characterized by excessive, involuntary and repetitive movements, which may involve the face, limbs or the entire body.
Through the program, eligible patients will receive a free supply of NITOMAN for six weeks. All patients will receive reimbursement assistance, if required, which will include insurance benefits and coverage verification, and prior authorization assistance.
Patient support services will include scheduled phone calls from registered pharmacists to encourage compliance with the prescribed dosing regimen and to discuss the potential side effects of therapy. Additionally, to help ensure that patients receive optimal benefit from treatment, pharmacists will collect information on dosing and treatment experience for feedback to patients' physicians.
Patients or their physicians who are interested in initiating the reimbursement assessment process should call the NITOMAN Access Hotline at 1-888-NITOMAN (1-888-648-6626). NITOMAN therapy will be provided to qualified Canadian patients who are in the process of obtaining either public or private coverage, and meet the approved indication for NITOMAN as well as the coverage criteria from public/private payors.
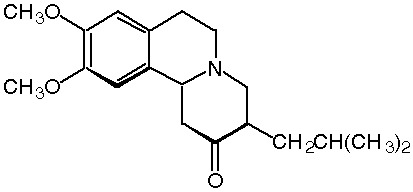
Tetrabenazine
In 2004, Prestwick Pharmaceuticals acquired the rights from Cambridge Laboratories Ltd., UK, to commercialize tetrabenazine in Canada under the brand name NITOMAN. Tetrabenazine is available in some European markets and New Zealand as XENAZINE(R). Tetrabenazine can have a profound effect on chorea, with many patients demonstrating marked to good improvement. Side effects can include drowsiness, insomnia, akathisia and depression.
Prestwick Pharmaceuticals
Prestwick Pharmaceuticals, Inc., is an emerging specialty pharmaceutical company that focuses on treatments for CNS disorders. The company has multiple product candidates in clinical development for Huntington's Disease, Parkinson's disease and schizophrenia. The company anticipates filing a New Drug Application (NDA) for tetrabenazine with the U.S. Food and Drug Administration (FDA) in the near future. Prestwick was granted both fast track and orphan designation by the FDA for tetrabenazine as a therapy for chorea associated with Huntington's Disease.
COPYRIGHT 2005 Business Wire
COPYRIGHT 2005 Gale Group