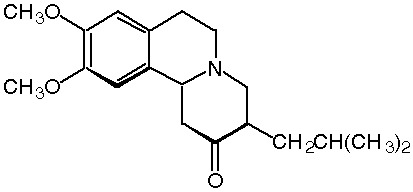
WASHINGTON -- Prestwick Pharmaceuticals, Inc., a CNS specialty pharmaceutical company, announced today the acquisition of rights to commercialize tetrabenazine in Canada under the brand name Nitoman(TM) from Cambridge Laboratories, a pharmaceutical company based in the United Kingdom (UK).
Tetrabenazine is a dopamine depletor approved in Canada for the treatment of a series of hyperkinetic movement disorders. Approximately 50,000 Canadians suffer from these disorders, which are characterized by excessive, involuntary purposeless and repetitive movements which may involve the face, limbs or the entire body.
Tetrabenazine is also approved for use in the UK, and a number of European countries, and Australia, and is currently undergoing Phase III pivotal trials in the United States (U.S.). Prestwick plans to file a New Drug Application (NDA) for tetrabenazine in the U.S. later this year.
"Acquiring the rights to Nitoman in Canada represents a significant commercial opportunity for Prestwick on a number of fronts," said David Cory, President and Chief Operating Officer of Prestwick Pharmaceuticals. "This move allows us to build infrastructure to support Canadian healthcare practitioners' and patients' use of Nitoman and begin to generate revenues for Prestwick as we conduct our Phase III program toward approval of tetrabenazine in the United States."
"Licensing commercial rights for Nitoman to Prestwick Pharmaceuticals makes good business sense and ensures that Nitoman will be freely available to patients in Canada who suffer from these disorders," said Mark Evans, Chief Executive Officer of Cambridge Laboratories. "This move allows Cambridge to focus on maximizing the full potential of tetrabenazine on a global basis, while Prestwick Pharmaceuticals manages the development and commercial growth of tetrabenazine in North America."
About Prestwick Pharmaceuticals
Prestwick Pharmaceuticals, Inc. is an emerging specialty pharmaceutical company that focuses on treatments for CNS disorders. The company has multiple product candidates in clinical development for chorea associated with Huntington's disease, Parkinson's disease, and schizophrenia. Prestwick plans to commercialize its lead product, tetrabenazine, in the U.S. as soon as approval is obtained from the U.S. Food and Drug Administration (FDA).
About Cambridge Laboratories
Cambridge Laboratories, based in Newcastle, UK, is a privately owned pharmaceutical company providing specialist products to serve the needs of the secondary healthcare physician. Cambridge's focus is in the areas of CNS, oncology, and niche hospital products. In 1997, Cambridge Laboratories acquired worldwide marketing rights for tetrabenazine.
COPYRIGHT 2004 Business Wire
COPYRIGHT 2004 Gale Group