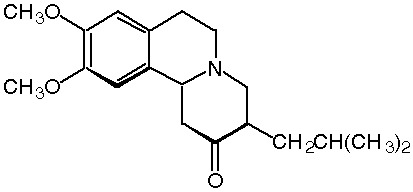
WASHINGTON -- Prestwick Pharmaceuticals, Inc., a CNS specialty pharmaceutical company, announced today that tetrabenazine has been designated as a "fast track" product by the U.S. Food and Drug Administration (FDA). Also, tetrabenazine has been designated as an orphan product by the FDA. Tetrabenazine is being evaluated for the treatment of chorea associated with Huntington's disease. Currently in pivotal clinical testing in the United States, tetrabenazine is a novel dopamine depletor that works by selectively blocking the VMAT2 transporter in the CNS.
The Fast Track Program is designed to expedite the review of drug compounds for the treatment of patients with serious or life-threatening diseases where there is an unmet medical need for new therapeutic approaches. The benefits of fast track designation provide for multiple meetings, timely comments, and a priority review at the FDA.
"This treatment addresses an unmet medical need, as there are currently no approved drugs for the treatment of chorea in Huntington's disease. The recent FDA action underscores the need for therapeutics for people with Huntington's Chorea and is most encouraging," said Kathleen Clarence-Smith, MD, PhD, Acting Chief Executive Officer, Prestwick Pharmaceuticals. "Prestwick is engaged in multiple programs to study the efficacy and safety of tetrabenazine in this patient population. We look forward to compiling the data generated from these trials and submitting it to the FDA."
Tetrabenazine is approved for use in several European countries and Australia as Xenazine(TM), and in Canada as Nitoman(TM).
About Prestwick Pharmaceuticals
Prestwick Pharmaceuticals, Inc. is an emerging specialty pharmaceutical company that focuses on treatments for CNS disorders. The company has multiple product candidates in clinical development for Huntington's Chorea, Parkinson's disease, and schizophrenia. Prestwick is currently conducting pivotal trials in the U.S. with its lead compound, tetrabenazine, and plans to file an NDA in the very near future.
COPYRIGHT 2004 Business Wire
COPYRIGHT 2004 Gale Group