Topamax
Topiramate (brand name: Topamax®) is an anticonvulsant drug produced by Ortho-McNeil, a division of Johnson & Johnson. It is used to treat epilepsy in both children and adults. In children it is also indicated for treatment of Lennox-Gastaut syndrome (a disorder that causes seizures and developmental delays). It is also FDA approved for, and now most frequently prescribed for, the prevention of migraines. It has been used by psychiatrists to treat bipolar disorder, although it is not FDA approved for this purpose and such use is somewhat controversial. more...
This drug has been investigated for use in treatment of obesity, especially to aid in the reduction of binge eating, and also as a possible treatment for alcoholism. However, these uses are not actively promoted by the manufacturer, and like its use for bipolar disorder, are "off-label" uses. The drug is also used in clinical trials to treat Post Traumatic Stress Disorder. A pilot study suggests that Topiramate is possibly effective against infantile spasm.
Pharmacodynamics
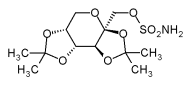
Chemically, Topiramate is a sulfamate-substituted monosaccharide, related to fructose, a rather unusual chemical structure for an anticonvulsant. Topiramate is quickly absorbed after oral use. Most of the drug (70%) is excreted in the urine as unchanged drug. The remainder is extensively metabolized by hydroxylation, hydrolysis, and glucuronidation. Six metabolites have been identified in humans, none of which constitutes more than 5% of an administered dose. Topiramate enhances GABA-activated chloride channels. In addition, Topiramate inhibits excitatory neurotransmission, through actions on kainate and AMPA receptors. There is evidence that Topiramate has a specific effect on GluR5 kainate receptors. It is also an inhibitor of carbonic anhydrase, particular subtypes II and IV, but this action is weak and unlikely to be related to its anticonvulsant actions, but may account for the bad taste and the development of renal stones seen during treatment. Its possible effect as a mood stabilizer seems to occur before anticonvulsant qualities at lower dosages. Topiramate inhibits maximal electroshock and pentylenetetrazol-induced seizures as well as partial and secundarily generalized tonic-clonic seizures in the kindling model, findings predective of a broad spectrum of antiseizure activities clinically.
Side effects
The most common side effects include a change in taste (carbonated beverages, especially diet sodas and beer, taste particularly bad) and feelings of pins and needles in the head and extremities. Less common side effects include cognitive deficiency (particularly word-finding difficulty); grogginess; lethargy; renal stones, impairment of fine motor skills; vision abnormality and transient or permanent vision loss (see below for FDA warning); weight loss; breast pain; abdominal pain; menstrual disorder; taste changes; pharyngitis; sinusitis; diplopia; rash; leukopenia; fatigue; dizziness; insomnia; anxiety; depression; paresthesia; diarrhea; nausea; dyspepsia; constipation; dry-mouth; dysmenorrhea.
Rarely, the inhibition of carbonic anhydrase may be strong enough to cause metabolic acidosis of clinical importance.
The side-effects most frequently leading to discontinuation of therapy with topiramate were :
Read more at Wikipedia.org