Studies reveal that surface waters worldwide are contaminated with hormonally active agents, many released from sewage treatment plants. Another potential source of aquatic hormonal contamination is livestock feedlot effluent. In this study, we assessed whether feedlot effluent contaminates watercourses by measuring a) total androgenic [methyltrienolone (R1881) equivalents] and estrogenic (17[beta]-estradiol equivalents) activity using the A-SCREEN and E-SCREEN bioassays and b) concentrations of anabolic agents via gas chromatography-mass spectroscopy and enzyme-based immunoassays. Water samples were collected over 3 years from up to six sites [all confluent with the Elkhorn River, Nebraska, USA: a feedlot retention pond (site 1), a site downstream from site 1 (site 2), a stream with intermediate livestock impact (site 3), and three sites with no observable livestock impact (sites 4-6)] and two sources of tap water. In 1999, samples from site 1 contained 9.6 pM R1881 equivalents and 1.7 pM 17[beta]-estradiol equivalents. Site 2 samples had estrogen levels similar to those in site 1 samples but lower androgen levels (3.8 pM R1881 equivalents). Androgen levels in site 3 samples were similar to those in site 2 samples, whereas estrogen levels decreased to 0.7 pM 17[beta]-estradiol equivalents. At site 6, androgen levels were approximately half those found at site 3, and estrogen levels were comparable with those at site 3. Sampling in later years was limited to fewer sites because of drought and lack of permission to access one site. Instrumental analysis revealed estrone but no significant levels of resorcylic acid lactones or trenbolone metabolites. Tap water was devoid of hormonal activity. We conclude that feedlot effluents contain sufficient levels of hormonally active agents to warrant further investigation of possible effects on aquatic ecosystem health. Key words: agricultural runoff, anabolic steroid hormones, aquatic ecosystem health, A-SCREEN, concentrated animal feeding operations (CAFOs), environmental androgens, environmental estrogens, E-SCREEN, personal care products, pharmaceuticals.
**********
In the 1990s, three sets of findings suggested that hormonally active agents may cause adverse health effects in humans and wildlife and thereby contribute to environmental degradation (Colborn et al. 1993). First, researchers discovered wildlife showing developmental, neurologic, and endocrine alterations, even after the bah of known estrogenic pesticides. Meanwhile, epidemiologic studies revealed increased incidences of breast and testicular cancers and alterations of the male genital tract (Davis et al. 1993; Sharpe and Skakkebaek 1993). Furthermore, chemicals introduced into the environment since 1950 were found to have hormone agonistic and antagonistic activity. It was hypothesized that health effects observed in humans and wildlife may be due to these endocrine-active chemicals, known as endocrine disruptors (Colborn et al. 1993). The E-SCREEN assay, developed to measure estrogenic activity using cell number as the end point (Soto et al. 1992, 1999), is used to identify estrogenic chemicals among environmental pollutants and is among the most sensitive and reproducible assays for estrogenic activity (Andersen et al. 1999; Fang et al. 2000).
Estrogen mimics were the majority of active agents first identified, although androgen agonists and antagonists were also found among environmental pollutants (Gray et al. 1999a, 1999b). These discoveries prompted the development of in vitro androgen agonist and antagonist assays such as the A-SCREEN (Soto et al. 1999).
Among more than 70,000 synthetic chemicals registered for commercial use, few were tested for hormonal activity. Because hormonal activity is not easily predictable, bioassays are necessary [Endocrine Disruptor Screening and Testing Advisory (EDSTAC) 1998]. In vitro rests were also adapted to detect estrogenic activity in wastewater and watercourses. Pairing water extract fractionation with instrumental analysis allowed for the identification of chemicals contributing to hormonal activity (Routledge et al. 1998). Fish exposed to these waters showed signs of endocrine disruption (Jobling et al. 1998).
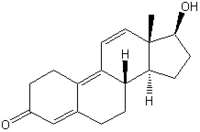
Anabolic agents are used in the cattle industry to increase growth. The androgens testosterone and trenbolone acetate (TbA), the estrogens 17[beta]-estradiol (17[beta]-[E.sub.2]) and zeranol, and the progestogens progestrerone and melengestrol acetate (MGA) (Lange et al. 2001) are the primary growth promoters used in the United States. Meat from anabolic steroid-treated cattle is purported to cause minimal human exposure because hormone treatment is stopped days before slaughter (Daxenberger et al. 2001). However, estrogenic esters of fatty acids, which are more potent than the parent compound when administered per os, have been identified in meat from anabolic steroid-treated cattle (Maume et al. 2001).
The ultimate fate of many excreted anabolic agents is unknown; however, measurable amounts of hormones are released from farm animals and reach the environment (Lange et al. 2002). Moreover, recent studies show that metabolites of TbA can remain in manure piles for more than 270 days, suggesting that these compounds are likely present downstream from cattle farms if excreta enter these waterways (Schiffer et al. 2001). In addition, estrogens and androgens have been reported in streams receiving runoff from fields fertilized with chicken litter (Finlay-Moore et al. 2000). Hence, we expect that both natural sex steroids in cattle and the metabolites of anabolic hormones administered to them would be present in the water downstream from cattle ranches.
In the present study, we tested the hypothesis that concentrated animal feeding operations (CAFOs) release significant amounts of natural hormones, anabolic steroids, and metabolites into the environment. The downstream hormonal contamination of such operations would potentially alter the reproductive and endocrine systems of exposed organisms.
Materials and Methods
Supplies. We purchased melengestrol from Biomol Research Laboratories (Plymouth Meeting, PA, USA); 17[beta]-[E.sub.2] from Calbiochem (San Diego, CA, USA); and methyltrienolone (R1881) from New England Nuclear (Perkin-Elmer Life Sciences, Boston, MA, USA). Francois Andre (LABERCA-National Reference Laboratory, Nantes, France) provided trenbolone-17[alpha] (Tb-17[alpha]), Tb-17[beta], and trendione (TbO). All other hormones were obtained from Sigma Chemical Company (St. Louis, MO, USA). Immunoaffinity chromatography columns for Tb and 19-nortestosterone (catalog no. TB 2186) were purchased from Randox Laboratories (San Diego, CA, USA).
Research sites. This study complements one by Orlando et al. (2004) on the endocrine-disrupting effects of cattle feedlot effluent on the fathead minnow, Pimephales promelas. Water was collected over a 3-year period from six sites confluent with the Elkhorn River in Nebraska (Figure 1). In June 1999, water samples were obtained from a cattle effluent holding pond directly below a feedlot (retention pond: site 1), a drainage canal 0.5 km downstream that channels water from the feedlot into the Elkhorn River (contaminated site: site 2), a stream draining fields with dispersed cattle and agricultural activity (intermediate exposure site: site 3), and three tributaries of the Elkhorn River with no apparent feedlot activity in the surrounding area (sites 4-6). Only one of these (reference site: site 6) yielded the proper sample size of fathead minnows required by Orlando et al. (2004) for their study. This site was located within the Oak Valley State Wildlife Management Area, approximately 80 km from the feedlot. In addition, tap water samples were acquired from the hose bibs of a recreation center (T1) and a recreational vehicle station center (T2) at a riverside park near site 3. Sampling in September 2000 and July 2001 was limited to sites 1, 3, and 6. Site 2 was not sampled after June 1999 because it was on private land and permission for additional samplings was not obtained. Site 3 was visited in both sampling periods, but drought prevented collection of water during September 2000.
[FIGURE 1 OMITTED]
Test sample preparation and extraction. Water from the retention pond and streams was collected by immersion of amber glass bottles: September 1999, 4-L bottles precleaned by U.S. Environmental Protection Agency (U.S. EPA) standards (Fisher Scientific, Pittsburgh, PA, USA); September 2000 and July 2001, precleaned 2.5-L bottles (catalog no. 2500-0250; Environmental Sampling Supply, Oakland, CA, USA). Tap water was collected from the hose bibs into the same bottles. We took from 8 L (1999) to 10 L (2000, 2001) from each site and added 0.02% sodium azide to avoid microbial degradation. Southwest Research Institute (SwRI; San Antonio, TX, USA) received the bottles and kept them at 4[degrees]C until extraction. SwRI prepared laboratory water blanks from distilled water samples.
We assessed the recovery of compounds likely to be in the field samples by spiking 2 L distilled water samples. Each analyte was spiked to 0.5 [micro]g/L, except for Tb, which was spiked to 2.5 [micro]g/L. We tried three extraction methods: dichloromethane (DCM), 6% ethyl ether-hexane, and 50% ethyl ether-hexane. Because DCM gave the best results, we used this for the field water samples.
Each sample was split into four 1-L fractions in 2-L glass separatory funnels. All glassware was rinsed with 0.6% HCI in DCM and allowed to dry. Each 1-L fraction was extracted three times with 60-mL portions of DCM by shaking 2 min and waiting for at least 10 min before draining the DCM into a 500-mL glass bottle. The DCM extracts of the four fractions were then combined into the same glass bottle. The DCM extracts were concentrated to 2 mL using nitrogen on an NEVAP evaporator (Organomation Associates, Berlin, MA, USA) maintained at 40[degrees]C. A 1-mL fraction was removed and solvent exchanged to 100 [micro]L ethanol. The remaining 1-mL fraction was submitted for gas chromatography-mass spectrometry (GC-MS) analysis as described below.
The spiked samples were solvent exchanged to acetonitrile and derivatized with N, Obis(trimethylsilyl)trifluoroacetamide (BSTFA; Regis Technologies, Morton Grove, IL, USA) for 4 hr at 70[degrees]C. Seven calibration standards, ranging from 15 to 1,000 ng/[micro]L, were prepared in acetonitrile and derivatized using BSTFA under the same conditions as samples before GC-MS analysis. External standard calibration was used to generate the response factors and to generate a first-order fit to check the linearity of the calibration curve.
Preparation of samples for Tb analysis by affinity chromatography. This procedure was used only for July 2001 field samples. We used Randox immunoaffinity columns (Randox Laboratories) for Tb/19-nortestosterone. Phosphate buffer was added to each 10-L field sample, and samples were passed through the columns following the supplier's protocol. A total volume of 1 L (900 mL of water sample and 100 mL phosphate buffer) was passed through the cartridge. Bound androgens were eluted with 4 mL 70% MeOH/30% water. The eluates were pooled, concentrated to 1 mL, and solvent exchanged to 100% ethanol. A 200-[micro]L aliquot was used for A-SCREEN and E-SCREEN assays, and an 800-[micro]L aliquot was used for HPLC separation and enzyme immunoassay (EIA).
Cell lines and culture conditions.
Maintenance and propagation of estrogen-target MCF7-BOS cells (Villalobos et al. 1995) and androgen-target MCF7-AR1 cells (Szelei et al. 1997) were performed as previously described (Soto et al. 1999). Charcoal-dextran stripping of fetal bovine serum (CDFBS) was performed as previously described (Soto et al. 1991).
E-SCREEN bioassay. Human breast cancer MCF7 cells are plated into 24-well plates (Linbro, ICN Biomedical, Costa Mesa, CA, USA) at an initial density of 20,000-30,000 cells/well in 1 mL Dulbecco's modification of Eagle's medium (DMEM; ICN Biomedical) supplemented with 5% fetal bovine serum (HyClone, Logan, UT, USA). The cells were dispersed evenly in each well and allowed to attach for 24 hr. The seeding medium was then replaced with sample extracts diluted with 5% CDFBS in phenol red-free DMEM (Irvine Scientific, Santa Ana, CA, USA).
Each experiment includes a 17[beta]-[E.sub.2] standard dose-response curve with 15 dilutions of 17[beta]-[E.sub.2] (0.05 pM-10.0 nM) in quadruplicate wells, run simultaneously with the samples. Results from the water sample extracts are interpolated into this dose-response curve.
Water extracts (100 [micro]L in ethanol) were prepared by adding 5% CDFBS to a final volume of 10 mL and tested at five different concentrations to ensure a cell number near the half-maximal level ([M.sub.50]) of the 17[beta]-[E.sub.2] dose-response curve. Water blanks and T1 and T2 samples were tested at 100-, 50-, 25-, 6.25-, and 1.25-fold greater than the initial water concentration. Field samples 1-6 were first tested at the same concentrations as the water blanks. Once the range of estrogenic activity had been established, a detailed concentration curve was run within that range to accurately measure estrogenic activity. On each 24-well plate we tested five concentrations from one extract in duplicate, plus negative (5% CDFBS) and positive controls (5% CDFBS plus 100 pM 17[beta]-[E.sub.2]). Assays for each water extract were repeated three to five times.
To evaluate potential cytotoxicity, each extract dilution was also tested in the presence of 100 pM 17[beta]-[E.sub.2] to induce maximal proliferation. If maximal proliferation was achieved, it was interpreted as a lack of toxicity. If the cell number was less than the positive control, it was used to qualify the results of that particular extract concentration as cytotoxic. In addition, cytotoxicity was identified through microscopic observation before fixation.
A-SCREEN bioassay. This assay uses MCF7-AR1 cells, which are stable transfectants of MCF7 cells expressing the wild-type human androgen receptor (Szelei et al. 1997). These cells proliferate maximally in 5% CDFBS and 100 pM 17[beta]-[E.sub.2], and respond to androgens by decreasing their proliferation rate. The assay compares the cell number of similar inocula of MCF7-AR1 cells grown in 5% CDFBS, 5% CDFBS plus 100 pM 17[beta]-[E.sub.2], and 5% CDFBS/100 pM 17[beta]-[E.sub.2] plus a range of concentrations of the synthetic, nonmetabolizable androgen R1881 (positive control) and a range of concentrations of a suspected androgen mimic (Soto et al. 1999). Each sample was assayed three to five times.
Field samples were analyzed by testing several dilutions of the ethanolic extract as described above for the E-SCREEN assay. The test samples, their corresponding field blanks, and an R1881 dose-response curve were processed simultaneously.
Processing for cell counting. We used fixation and staining techniques previously described by Villalobos et al. (1995). The bound sulforhodamine B (SRB) dye was solubilized using 500 [micro]L 10 mM Tris base (pH 10.5) per well; triplicate 100-[micro]L aliquots were transferred to 96-well plates (Becton Dickinson, Franklin Lanes, NJ, USA) and scanned in a computerized microplate reader (Series 750; Cambridge Technology, Inc., Watertown, MA, USA) at 515 nm wavelength. The relationship between optical density and cell number was established by comparing different cell inocula and counting hall of the wells with a Coulter counter (Coulter Electronics, Hialeah, FL, USA) and half via the SRB method.
Data analysis. 17[beta]-[E.sub.2] and R1881 dose-response curves were used as standards to quantify estrogenic and androgenic activity of samples in 17[beta]-[E.sub.2] equivalents ([E.sub.2]Eq) or R1881 equivalents (AEq). We expressed the cell number corresponding to each 17[beta]-[E.sub.2] (or R1881) concentration relative to the maximal cell number from the experiment with the following equation:
B/[B.sub.0] = cell number of curve point - cell number of negative control/maximal cell number - cell number of negative control
We then logit-transformed the B/[B.sub.0] ratio and ran a simple linear regression of logit B/[B.sub.0] on the natural log of the 17[beta]-[E.sub.2] (or R1881) concentration and graphed the linearized curve. We excluded points with B/[B.sub.0] < 0.15 or > 0.85, because these points fall near the asymptotes (< 0.15, below detection limits; > 0.85, in the plateau) where the relationship between dose and effect is meaningless (Feldman and Rodbard 1971). The slope and y-intercept of the linear logit-transformed dose-response curve were then used to calculate the [E.sub.2]Eq or AEq corresponding to B/[B.sub.0] for any tested samples.
Conversion of analytical data into hormone equivalent units. [M.sub.50] is the concentration producing an estrogenic (of androgenic) effect that is 50% of the maximal response. This parameter is measured to assess the potency of agonists, relative to the standard. The relative proliferative potencies (RPPs) were calculated as ([M.sub.50] 17[beta]-[E.sub.2] or R1881) / ([M.sub.50] test chemical). Based on the mean [M.sub.50], the "predicted" estrogen/androgen total load was calculated and compared with the actual values obtained by measuring bioactivity with the E-SCREEN and A-SCREEN assays.
All target analytes [estrone ([E.sub.1]), 17[beta]-[E.sub.2], Tb-17[alpha], Tb-17[beta], the six resorcylic acid lactones ([alpha]-zearalenol, [beta]-zearalenol, [alpha]-zearalanol, [beta]-zearalanol, zearalenone, zearalanone), and MGA] were assayed using both the E-SCREEN and the A-SCREEN assays.
Immunometric detection of estrogens and androgens. Water extracts (100-[micro]L aliquots) were diluted with purified water (Millipore-Milli-Q-PLUS Purification Pak CPMQ004R1; Millipore Corp., Billerca, MA, USA) to a final ethanol concentration of 20% and passed through 100-mg octadecyl silica gel cartridges (Bakerbond solid-phase extraction [C.sub.18]; J.T. Baker Inc., Phillipsburg, NJ, USA). Cartridges were washed with 2 x 1 mL methanol and equilibrated with 2 x 1 mL 20 mM Tris-HCl (pH 8.5)/methanol 80/20 (vol/vol). After sample application, the cartridge was washed twice each with 1 mL 20 mM Tris-HCl (pH 8.5)/methanol 80/20 (vol/vol) and 40% aqueous methanol. Hormones were eluted with 1 mL 80% aqueous methanol.
The solvent was evaporated at 60[degrees]C under reduced pressure (3 hPa; Unijet II, Uni Equip, Munich, Germany). For HPLC analysis, the residue was redissolved in 300 [micro]L 20 mM Tris-acetate (pH 7.2)/acetonitrile 80/20 (vol/vol) for [E.sub.2] or 300 [micro]L purified water/methanol 80/20 (vol/vol) for Tb. According to previously established procedures (Lange et al. 2001; Schiffer et al. 2001), the extracts were processed via HPLC and prepared for analysis by specific immunometric methods (EIA). The estrogen and Tb fractions were analyzed by EIA as previously described (Meyer and Hoffmann 1987; Meyer et al. 1997).
GC-MS detection of anabolic agents. For low-resolution measurements of anabolic agents, we used an Agilent 5973 single-quadrupole mass spectrometer (Palo Alto, CA, USA), coupled with an Agilent 6890 gas chromatograph used with an Agilent DB-5.625 (30 mm x 0.25 mm inner diameter x 0.25 [micro]m) analytical column (inlet temperature, 275[degrees]C; injection volume, 2 [micro]L; J&W Scientific, Folsom, CA, USA). The injection mode was pulsed splitless with a 1-min purge time and a 50 mL/min purge flow. The carrier gas was helium. The oven temperature was programmed for 60[degrees]C (1 min) and 8[degrees]C/min to 310[degrees]C (5 min), and the transfer line temperature was 280[degrees]C. Temperatures were set at 150[degrees]C and 230[degrees]C for the quadrupoles and source, respectively. We used electron impact (EI) as the ionization technique. Mass spectra data were acquired under selected ion monitoring (SIM) mode in which three to four ions per compound were scanned to enhance sensitivity.
For high-resolution measurements, the ethanolic extracts were further purified for GC-high resolution MS (GC-HRMS) through three solid-phase extraction columns ([C.sub.18], diol, and silica) (Maume et al. 2001). The derivatization reagents included n-methyl-n-trimethylsilyl-trifluoroacetamide, and trimethyliodosilane (Fluka, Buchs, Switzerland). The measurements were obtained as described by Maume et al. (2001) using EI ionization and SIM acquisition with perfluorokerosene providing the "lock" mass and 1,3,5(10)-estratriene-16, 16,17-[d.sub.3]-17[beta]-diol providing the internal standard.
Results
Choice of extraction method, Neither 6% nor 50% ethyl ether-hexane gave acceptable recoveries. Extraction with DCM gave acceptable recoveries for all the analytes of interest: 102% for estrone ([E.sub.1]), 87% for 17[beta]-[E.sub.2], 99% for testosterone, 103% for Tb-17[beta], 100% for Tb-17[beta], and between 95% and 101% for six resorcylic acid lactones.
Hormonal activity of anabolic compounds. The [M.sub.50] values for the resorcylic acid lactones were as follows: [alpha]-zearalenol, 50 pM; [beta]-zearalenol, 4.3 nM; [alpha]-zearalanol, 0.13 nM; [beta]-zearalanol, 0.6 nM; zearalenone, 0.94 nM; and zearalanone, 0.43 nM, compared with 7.5 pM for 17[beta]-[E.sub.2]. Androgens (up to 1 [micro]M) had no estrogenic activity.
The [M.sub.50] of the isomers Tb-17[alpha] and Tb-17[beta] were 2.35 and 0.15 nM, respectively; the latter had similar potency to dihydro-testosterone ([M.sub.50], 75 pM) and R1881 ([M.sub.50], 68.4 pM). MGA had neither estrogenic nor androgenic activity.
Dose-response curves for 17[beta]-[E.sub.2] and R1881. The [M.sub.50] values were 7.5 [+ or -] 2.1 pM for 17[beta]-[E.sub.2] (n = 9) and 68.4 [+ or -] 23.1 pM for R1881 (n = 7). The [R.sup.2] values for 17[beta]-[E.sub.2] (n = 9) and R1881 (n = 7) were 0.97 [+ or -] 0.04 and 0.97 [+ or -] 0.03, respectively. Figure 2 depicts the 17[beta]-[E.sub.2] and R1881 dose-response curves.
[FIGURE 2 OMITTED]
Estrogen and androgen activity in the field samples. For June 1999 field samples, both estrogen and androgen activities were highest in site 1 samples. There was a marked decline of androgen activity in site 2 samples, comparable with that in samples from sites 3-5. Site 6 samples exhibited a lower activity, about 45-50% that of samples from sites 2-5 and about 25% that of site 1 samples; androgen activity of samples was as follows (in picomoles AEq): site 1, 9.62 [+ or -] 1.5%; site 2, 3.83 [+ or -] 14%; site 3, 4.58 [+ or -] 1%; site 4, 3.89 [+ or -] 14%; site 5, 4.58 [+ or -] 7.2%; and site 6, 2.45 [+ or -] 16%. In contrast, estrogenic activity was similar at sites 1 and 2 and roughly 50% lower at site 6 (Figure 3); estrogenic activity of samples was as follows (in picomoles [E.sub.2]Eq): site 1, 1.73 [+ or -] 6.2%; site 2, 2.23 [+ or -] 17%; site 3, 0.65 [+ or -] 1.5%; site 4, 0.78 [+ or -] 16%; site 5, 0.76 [+ or -] 19%; and site 6, 1.15 [+ or -] 19%. No estrogenic or androgenic activity was found in drinking water (T1 and T2) of in the water blanks. Detection of estrogens and androgens by EIA and GC-MS was problematic because of the low signal-to-noise ratio. We tested for Tb-17[beta] and its metabolites Tb-17[alpha] and TbO, as well as zeranol, 17[beta]-[E.sub.2], 17[alpha]-[E.sub.2], and [E.sub.1]. The only analyte measurable in these conditions of extremely high noise was [E.sub.1] (Table 1). There was a 6-fold difference between [E.sub.1] activities at sites 1 (1,650 pg/L) and 6 (270 pg/L); [E.sub.1] activity at site 2 was 354 pg/L. [E.sub.1] was below detection limits in T1 and T2 samples. The detection limit for these two samples was 1-3 pg/L, two orders of magnitude below the levels found in field samples. These values were confirmed by GC-HRMS. [E.sub.2]-17[alpha] was detected only at site 5 (19 pg/L).
[FIGURE 3 OMITTED]
We measured the RPP of [E.sub.1] (relative to 17[beta]-[E.sub.2]) to assess the proportion of estrogenic activity detected in the E-SCREEN assay that is due to [E.sub.1] (RPP, 0.0436). The estrogenic activity of [E.sub.1] accounted for a variable portion (between 3% and 46%) of estrogenic activity found by the E-SCREEN assay (Table 1).
To identify the analytes responsible for the hormonal activity, we collected samples again in September 2000. We detected both androgenic and estrogenic activity (Table 2). Detection of Tb and zeranol was thwarted because of high noise. [E.sub.1] was positive (8,300 pg/L) and represented 36.34% of the oral estrogenic activity detected by the E-SCREEN (Table 3). 17[alpha]-[E.sub.2], 17[beta]-[E.sub.2], and [E.sub.1] were also analyzed by GC-HRMS, yielding comparable results (Table 3). These results suggest that other estrogens in addition to our target analytes could have been present.
To decrease noise and enable the detection of Tb and its metabolites in July 2001 field samples, we chromatographed water samples taken from sites 1, 3, and 6 through Tb/19-nortestosterone immunoaffinity columns. The A-SCREEN revealed 1.37, 0.5, and 0.19 pM AEq at sites 1, 3, and 6, respectively, and no activity in the water blanks. However, the purification yield was not measured because a tracer would interfere with the bioactivity assays. As expected, the E-SCREEN assay detected no estrogenic activity in these supposedly androgen-only preparations (Table 2). Tb-17[beta], Tb-17[alpha], and TbO were detected, albeit at concentrations about 100-fold lower than that needed for androgen detection by the A-SCREEN (Table 4). The extremely low level at site 1 (theoretically the most polluted), and the low but detectable levels of Tb-17[alpha] and TbO in the water blanks suggest that these data may be artifactual. Water blanks processed later revealed undetectable levels of the three Tb-related analytes.
Discussion
The recent increase in the number of CAFOs raises concerns that their wastewater may contaminate downstream watercourses and thereby contribute to environmental degradation. According to the new U.S. EPA ruling (U.S. EPA 2003), more CAFOs will be required to seek discharge permits under the Clean Water Act (1970) and to develop and implement nutrient management plans.
Our original purpose was to compare hormonal activities and profiles in the runoff from CAFOs that supplement their cattle with hormones with those in runoff flora CAFOs that do not. We were unable to identify any sites where animals were raised without hormone supplements in a feedlot setting. In hindsight, this is not surprising because hormone supplements are given to approximately 90% of U.S. beef cattle (Balter 1999). Cattle raised without hormone supplements are usually raised at low density on open rangeland; hence, their excreta should present a substantially lower environmental borden.
The hormones are usually given in the form of implants; the implants currently marketed contain pharmaceuticals with androgenic (testosterone, TbA), estrogenic (17[beta]-[E.sub.2], zeranol), or progestogenic (MGA) activities, and deliver either single hormones [e.g., Finaplix-H (Intervet, Millsboro, DE, USA), 200 mg TbA Ralgro (Mallinckrodt Veterinary, Mundlein IL, USA), 36 mg zeranol] of a mixture [e.g. Synovex-H (Fort Dodge Animal Health Wyeth, Madison, NJ, USA), 200 mg testosterone propionate plus 20 mg [E.sub.2] benzoate (Lange et al. 2001). The progestogen agonist MGA was devoid of estrogenic and androgenic activity; however, Meyer (2001) reported that administering MGA to female cattle increases their plasma [E.sub.2] levels.
We collected runoff water from feedlots where animals were treated with anabolic steroids. A significant portion of these steroids and their metabolites are excreted as conjugates (Schiffer et al. 2001), which are not extracted by DCM. However, conjugates are metabolized by bacteria into their DCM-extractable free form in sewage and surface waters (Irwin et al. 2001). Hence, the results presented here may underestimate actual exposure if conjugates were present in the water. We did not know a priori what type of hormone implant was used in this feedlot and were not able to obtain this information upon request. Therefore, we first measured the total estrogenic and androgenic activity at different points downstream of one such operation to assess the exposure of fish (Orlando et al. 2004). We then analyzed the water extracts to assess the presence of pharmaceuticals frequently used as anabolic steroids (this study).
Bioassays measure the total activity of mixtures of chemicals that act through the same receptor systems (Silva et al. 2002); activity is expressed in concentration units of the standard (Soto et al. 1997). These data are then contrasted with instrumental analyses of the sample. Ideally, the analytes measured account for the total hormonal activity found in the bioassay. The presence of additional active compounds in the mixture is suggested when the instrumental analysis reveals a lower theoretical activity than the bioassay detects.
Androgenic activity. The total androgenic activity measured by A-SCREEN seems to have originated in the feedlots, because it was highest at site 1 and decreased to < 40% at site 2, located 0.5 km downstream. The intermediate contamination site, which also drained feedlots, had androgenic activity comparable with that of site 2, which was 2-fold higher than in the reference site. At the time of sample collection, sites 4 and 5 were believed to be free of feedlot exposure. The hormonal activity in the reference sites could be a result of manure water slurry that had been sprayed on crops in the vicinity, which we learned of after the water samples had been collected, processed, and assayed.
Marginal levels of Tb and its metabolites (representing 0.1-1.1% of the total androgenic activity) were detected. Hence, the androgenic activity may be attributed to natural androgens. The octanol-water partition coefficient of natural androgens (3.3 for testosterone) suggests a potential for sorption to organic matter and, thus, a higher concentration in sediment (not analyzed) than in water.
Estrogenic activity. Remarkably, estrogenic activities at sites 1 and 2 would be sufficient to produce a significant effect on target cells. Resorcylic acid lactones were not detected; instead, [E.sub.1] was detected in all sites and represented up to 46% of the total estrogenic activity. 17[alpha]-[E.sub.2] and 17[beta]-[E.sub.2] were barely detectable in some samples. The main metabolite of 17[beta]-[E.sub.2] in cattle excreta is 17[alpha]-[E.sub.2]. We were not surprised, however, to find that the main estrogen detected was [E.sub.1]. It has been established that microorganisms in the environment degrade estrogens and that [E.sub.2] is rapidly transformed to [E.sub.1] in river water and in sediments (Jurgens et al. 2002). The log octanol--water partition coefficients of [E.sub.2] and [E.sub.1] are reported to be in the range of 3-4, indicating sorption potential to organic matter. Therefore, higher concentrations of estrogens may be present in the sediment. This is especially important concerning [E.sub.1], which is persistent (Environment Agency 2002). The fact that [E.sub.1] did not account for all the estrogenic activity suggests that estrogenic compounds other than those analyzed may have been present. DCM also extracts pesticides, plasticizers, and other xenoestrogens. We detected diethylphthalate (2 ng/L), atrazine (6 ng/L), metolachlor (3.3 ng/L), and cyanazine (1.1 ng/L) in some of the feedlot samples, but not in drinking water. Diethylphthalate is estrogenic (Harris et al. 1997), and although some commonly used herbicides (i.e., atrazine) are not estrogenic, they do appear to activate aromatase and thus increase estrogen production in alligators (Crain et al. 1997) and frogs (Hayes et al. 2002) and in some fish and mammalian cell lines (Sanderson et al. 2001).
It is worth noting that water samples taken in July 2002 and processed by affinity chromatography to purify Tb were devoid of estrogenic activity. This shows that the androgens present in these preparations do not produce false positives when tested by the E-SCREEN assay and it also confirms our prior results (Soto et al. 1998; Soto et al. 1999). Drinking water from wells showed neither estrogenic nor androgenic activity.
In summary, these data indicate that significant estrogenic and androgenic activity is released into water by feedlot operations. Our findings are compatible with the hypothesis that the animals in this feedlot may have been treated with an anabolic mixture of androgen and estrogen, such as testostetone propionate and estradiol benzoate.
These data support the findings of Orlando et al. (2004) regarding masculinization of female fish captured at sites 2 and 3. This effect could be a consequence of androgen exposure (at least 2-fold higher at sites 2 and 3 than at site 6), and the demasculinization found at these two sites may be due to the combined effect of estrogens and androgens, which may have altered the regulation of gonadotropin-releasing factors and/or gonadotropins in the exposed fish. The fact that fish are affected suggests the need for future studies to further examine the mechanisms leading from exposure to the observed effects. On one hand, difficulties regarding the isolation of natural hormones and anabolic steroids require new purification protocols and sensitive analytical techniques for assessing exposure. On the other hand, our data highlight the usefulness of the E-SCREEN and A-SCREEN assays because they allow the estimation of total estrogen and androgen exposure of fish and other wildlife using a single-step, easily performed extraction method.
These findings contribute to the increasing literature documenting the contamination of water bodies in the United States and Europe with numerous bioactive agents, including natural hormones, xenohormones, and pharmacologic agents (Allen et al. 1999; Kolpin et al. 2002; Vethaak et al. 2002), and point to the urgent need to take steps to curtail environmental degradation caused by the release of these chemicals into bodies of water.
We thank D. Maume for help with high resolution GS-MS estrogen measurement methodology and C. Michaelson and C. Wieloch for skilled technical assistance.
This work was supported by the European Commission (contracts BIO4-CT98-4757 and FAIR-CT98-4752). The authors declare they have no competing financial interests.
REFERENCES
Allen Y, Scott AP, Matthiessen P, Haworth S, Thain JE, Feist S. 1999. Survey of estrogenic activity in United Kingdom estuarine and coastal waters and its effects in gonadal development of the flounder Platichthys flesus. Environ Toxicol Chem 18:1791-1800.
Andersen HR, Andersson AM, Arnold SF, Autrup H, Barfoed M, Beresford NA, et al. 1999. Comparison of short-term estrogenicity tests for identification of hormone-disrupting chemicals. Environ Health Perspect 107(suppl 1):89-108.
Balter M. 1999. Scientific cross-claims fly in the continuing beef war. Science 284:1459-1455.
Clean Water Act 1970. 42USC7401.
Colborn T, vom Saal FS, Soto AM. 1993. Developmental effects of endocrine-disrupting chemicals in wildlife and humans, Environ Health Perspect 101:379-384.
Crain DA, Guillette LJ Jr, Rooney AA, Pickford DB. 1997. Alterations in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ Health Perspect 105:528-533.
D'Ascenzo G, Di Corcia A, Gentili A, Mancini R, Mastropasqua R, Nazzari M, et al. 2003. Fate of natural estrogen conjugates in municipal sewage transport and treatment facilities. Sci Total Environ 302:199-209.
Davis DL, Bradlow HL, Wolff M, Woodruff T, Hoel DG, Anton-Culver H. 1993. Medical hypothesis: xenoestrogens as preventable causes of breast cancer. Environ Health Perspect 101:372 377.
Daxenberger A, Ibarreta D, Meyer HHD. 2001. Possible health impact of animal oestrogens in food. Hum Reprod Update 7:340-355.
EDSTAC. 1998. Endocrine Disruptor Screening and Testing Advisory Committee (EDSTAC) Final Report. Washington, DC:U.S. Environmental Protection Agency.
Environment Agency. 2002. Proposed Predicted-No-Effect-Concentrations (PNECs) for Natural and Synthetic Steroid Oestrogens in Surface Waters. Technical Report P2-TO4/1/TR. Bristol, UK:Environment Agency.
Fang H, Tong W, Perkins R, Soto AM, Prechtl NV, Sheehan DM. 2000. Quantitative comparisons of in vitro assays for estrogenic activities. Environ Health Perspect 108:723-729.
Feldman H, Rodbard D. 1971. Mathematical theory of radio-immunoassay. In: Principles of Competitive Protein-Binding Assays (Odell WB, Daughaday WH, eds). Philadelphia:J.B. Lippincot Co., 159-199.
Finlay-Moore O, Hartel PG, Cabrera ML. 2000, 17[beta]-Estradiol and testosterone in soil and runoff from grasslands amended with broiler litter. J Environ Qual 29:1604-1611
Gray LE Jr, Ostby J, Cooper RL, Kelce WR. 1999. The estrogenic and antiandrogenic pesticide methoxychlor alters the reproductive tract and behavior without affecting pituitary size or LH and prolactin secretion in male rats. Toxicol Ind Health 15:37-47.
Harris CA, Henttu P, Parker MG, Sumpter JP. 1997. The estrogenic activity of phthalate esters in vitro. Environ Health Perspect 105:802-811.
Hayes TB, Collins A, Lee M, Mendoza M, Noriega N, Stuart AA, et al. 2002. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low, ecologically relevant doses. Proc Natl Acad Sci USA 99:5476-5479.
Howell WM, Black DA, Bortone SA. 1980. Abnormal expression of secondary sex characters in a population of mosquito-fish, Gambusia affins holbrooki. evidence from environmentally-indiced masculinization. Copeia 4:676-681.
Irwin LK, Gray S, Oberdorster E. 2001. Vitellogenin induction in painted turtle, Chrysemys picta, as a biomarker of exposure to environmental levels of estradiol. Aquat Toxicol 55:49-60.
Jobling S, Nolan M, Tyler CR, Brighty G, Sumpter JP. 1998. Widespread sexual disruption in wild fish. Environ Sci Technol 32:2498-2506.
Jurgens MD, Holthaus KIE, Johnson AC, Smith JJL, Hetheridge M, Williams RJ. 2002. The potential for estradiol and ethinylestradiol degradation in English rivers. Environ Toxicol Chem 21:480-488.
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, et al. 2002. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999-2000: a national reconnaissance. Environ Sci Technol 36:1202-1211.
Lange IG, Daxenberger A, Meyer HHD. 2001. Hormone contents in peripheral tissues after correct and off-label use of growth promoting hormones in cattle: effect of implant preparations Finaplix-H, Ralgro, Synovex-H, and Synovex Plus. APMIS 109:53-65.
Lange IG, Daxenberger A, Schiffer B, Witters H, Ibarreta D, Meyer HHB. 2002. Sex hormones originating from different livestock production systems: fate and potential disrupting activity in the environment. Anal Chim Acta 473:27-37.
Maume D, Deceuninck Y, Pouponneau K, Paris A, Le Bizec B, Andre F. 2001. Assessment of estradiol and its metabolites in meat. APMIS 109:32-39.
Meyer HHD, 2001. Biochemistry and physiology of anabolic hormones used for improvement of meat production. APMIS 109:1-8.
Meyer HHD, Hoffmann S. 1987. Development of a sensitive microtitration plate enzyme-immunoassay for the anabolic steroid trenbolone. Food Addit Contam 4:149-160.
Meyer HHD, Rohleder M, Streich WJ, Goltenboth R, Ochs A. 1997. Sex steroid profiles and ovarian activities of the female panda Yan Yan in the Berlin Zoo [in German]. Berl Munch Tierarztl Wochenschr 119:143-147.
Orlando EF, Kolok AS, Binzcik GA, Gates JL, Horton MK, Lambright CS, et al, 2004. Endocrine-disrupting effects of cattle feedlot effluent on an aquatic sentinel species, the fathead minnow. Environ Health Perspect 112:353-358.
Routledge EJ, Sheahan D, Desbrow C, Brighty GC, Waldock M, Sumpter JP. 1998. Identification of estrogenic chemicals in STW effluent. 2. In vivo responses in trout and roach. Environ Sci Technol 132:1559-1565.
Sanderson JT, Letcher RJ, Heneweer M, Geisey JP, van den Berg M. 2001. Effects of chloro-s-triazine herbicides and metabolites on aromatase activity in human cell lines and on vitellogenin in male carp hepatocytes. Environ Health Perspect 109:1027-1031.
Schiffer B, Daxenberger A, Meyer K, Meyer HHD. 2001. The fate of trenbolone acetate and melengestrol acetate after application as growth promoters in cattle: environmental studies. Environ Health Perspect 109:1145-1511.
Sharpe RM, Skakkebaek NE. 1993, Are oestrogens involved in falling sperm count and disorders of the male reproductive tract? Lancet 341:1392-1395.
Silva E, Rajapakse N, Kortenkamp A. 2002, Something from "nothing" eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ Sci Technol 36:1751-1756.
Soto AM, Fernandez MF, Luizzi MF, Oles Karasko AS, Sonnenschein C. 1997. Developing a marker of exposure to xenoestrogen mixtures in human serum. Environ Health Perspect 105:647-654.
Soto AM, Justicia H, Wray JW, Sonnenschein C. 1991. p-Nonylphenol: an estrogenic xenobiotic released from "modified" polystyrene. Environ Health Perspect 92:167-173.
Soto AM, Lin T-M, Justicia H, Silvia RM, Sonnenschein C. 1992. An "in culture" bioassay to assess the estrogenicity of xenobiotics. In: Chemically-Induced Alterations in Sexual Development: The Wildlife/Human Connection (Colborn T, Clement C, eds). Princeton, NJ:Princeton Scientific Publishing, 295-309.
Soto AM, Michaelson CL, Prechtl NV, Weill BC, Sonnenschein C. 1999. In vitro endocrine disruptor screening. In: Environmental Toxicology and Risk Assessment, Vol 8 (Henshel D, ed). ASTM STP 1364. West Conshohocken, PA:American Society for Testing and Materials, 39-59.
Soto AM, Michaelson CL, Prechtl NV, Weill BC, Sonnenschein C, Olea-Serrano MF, et al, 1998. Assay to measure estrogen and androgen agonists and antagonists. In: In Vitro Germ Cell Developmental Toxicology: From Science to Social and Industrial Demand (del Mazo J, ed). New York:Plenum Press, 9-28.
Szelei J, Jimenez J, Soto AM, Luizzi MF, Sonnensehein C. 1997. Androgen-induced inhibition of proliferation in human breast cancer MCF7 cells transfected with androgen receptor. Endocrinology 138:1406-1412.
U.S. EPA (U.S. Environmental Protection Agency). 2003, National Pollution Discharge Elimination System Permit Regulation and Effluent Limitation Guidelines and Standards for Concentrated Animal Feeding Operations (CAFOs); Final Rule. Fed Reg 68:7175-274.
Vethaak AD, Rijs GBJ, Schrap SM, Ruiter H, Gerritsen A, Lahr J. 2002. Estrogens and Xeno-estrogens in the Aquatic Environment of the Netherlands. Occurrence, Potency and Biological Effects. Riza/RikZ Report no. 2002.001. Lelystad, Netherlands:Ministry of Transport, Public Works and Water Management. Available: http://www.minvenw.nl/rws/riza/home/publicaties/riza_rapporten /pdf_rapport/rr_2002_0O1.pdf? [accessed 16 January 2004].
Villalobos M, Olea N, Brotons JA, Olea-Serrano MF, Ruiz de Almodovar JM, Pedraza V. 1995. The E-screen assay: a comparison of different MCF7 cell stocks. Environ Health Perspect 103:844-850.
Ana M. Soto, (1) Janine M. Calabro, (1) Nancy V. Prechtl, (7) Alice Y. Yau, (2) Edward F. Orlando,3 Andreas Daxenberger, (4) Alan S. Kolok, (5) Louis J. Guillette, Jr., (6) Bruno le Bizec, (7) Iris G. Lange, (4) and Carlos Sonnenschein (1)
(1) Department of Anatomy and Cellular Biology, Tufts University School of Medicine, Boston, Massachusetts, USA; (2) Southwest Research Institute, San Antonio, Texas, USA; (3) Biology Department, St. Mary's College of Maryland, St. Mary's City, Maryland, USA; (4) Institut fur Physiologie, Forschungszentum fur Milch und Lebensmittel, Technischen Universitat Munchen-Weihenstephan, Freising-Weihenstephan, Germany; (5) Department of Biology, University of Nebraska at Omaha, Omaha, Nebraska, USA; (6) Department of Zoology, University of Florida, Gainesville, Florida, USA; (7) LABERCA-Laboratoire National de Reference, Ecole Nationale Veterinaire de Nantes, Nantes, France
Address correspondence to A.M. Soto, Department of Anatomy and Cellular Biology, Tufts University School of Medicine, 136 Harrison Ave., Boston, MA 02111 USA. Telephone: (617) 636-6954. Fax: (617) 636-3971. E-mail: ana.soto@tufts.edu
Received 14 July 2003; accepted 1 December 2003.
COPYRIGHT 2004 National Institute of Environmental Health Sciences
COPYRIGHT 2004 Gale Group