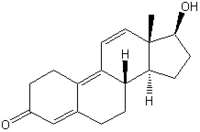
The Organisation for Economic Co-operation and Development has initiated the development of new guidelines for the screening and testing of potential endocrine disruptors. The Hershberger assay is one of the assays selected for validation based on the need for in vivo screening to detect androgen agonists or antagonists by measuring the response of five sex accessory organs and tissues of castrated juvenile male rats: the ventral prostate, the seminal vesicles with coagulating glands, the levator ani and bulbocavernosus muscle complex, the Cowper's glands, and the glans penis. The phase 1 feasibility demonstration stage of the Hershberger validation program has been successfully completed with a single androgen agonist and a single antagonist as reference substances. The phase 2 validation program employs a range of additional androgen agonists and antagonists as well as 5[alpha]-reductase inhibitors. Seven Japanese laboratories have contributed phase 2 validation studies of the Hershberger assay using methyltestosterone, vinclozolin, and 2,2-bis (4-chlorophenyl)-1,1-dichloroethylene (p,p'-DDE). The methyltestosterone doses were 0, 0.05, 0.5, 5, and 50 mg/kg/day, and the vinclozolin and p,p'-DDE doses were 0, 3, 10, 30, and 100 mg/kg/day. All chemicals were orally administered by gavage for 10 consecutive days. In the antagonist version of the assay using vinclozolin and p,p'-DDE, 0.2 mg/kg/day of testosterone propionate was coadministered by subcutaneous injection. All five accessory sex preproductive organs and tissues consistently responded with statistically significant changes in weight within a narrow window. Therefore, the Japanese studies support the Hershberger assay as a reliable and reproducible screening assay for the detection of androgen agonistic and antagonistic effects. Key words: Hershberger assay, methyltestosterone, OECD validation, p,p'-DDE, vinclozolin. Environ Health Perspect 111:1912-1919 (2003). doi:10.1289/ehp.6357 available via http://dx.doi.org/ [Online 10 September 2003]
**********
Certain reproductive and developmental toxicants may have the potential to interfere with normal sexual differentiation and development in animals and humans by modulating or interfering with the endocrine system (McLachlan 1993; McLachlan and Korach 1995). The Organisation for Economic Cooperation and Development (OECD) has initiated an activity, to revise existing guidelines and develop new screening and testing guidelines to aid in the identification and assessment of such toxicants (OECD 1998, 2000, 2001, 2003).
One proposed assay, referred to as the Hershberger assay,, uses the androgen sensitivity of several accessory sex organs and tissues of the male reproductive tract. The assay was originally developed in the 1930s by Korenchevsky. and co-workers, and a number of accessory sex organs and tissues were shown to be useful by these and other investigators, including the ventral prostate (Deanesly and Parkes 1936; Dingemanse et al. 1935; Korenchevsky 1932; Korenchevsky et al. 1932, 1933a, 1933b), the seminal vesicles and coagulating glands (Deanesly and Parkes 1936; Dingemanse et al. 1935; Korenchevsky 1932; Korenchevsky et al. 1932, 1933a, 1933b), the preputial glands (Bulbring and Burn 1935; Korenchevsky 1932; Korenchevsky et al. 1932, 1933a, 1933b), the Cowper's glands (Wainman and Shipounoff 1941), and the glans penis (Bulbring and Burn 1935; Dingemanse et al. 1935; Korenchevsky 1932; Korenchevsky et al. 1932, 1933a, 1933b). In the 1940s, it was discovered that the levator ani and bulbocavernosus muscles also responded to androgens, but in a different way from the other tissues (Eisenberg and Gordan 1950; Eisenberg et al. 1949; Wainman and Shipounoff 1941). The basis for this differential sensitivity is the presence of 5[alpha]-reductase in most accessory tissues of the male reproductive tract but its absence in the muscle complex (Di Salle et al. 1994). The capabilities of the assay were demonstrated in 1953 by Hershberger et al. when they analyzed the response of the ventral prostate, seminal vesicles and coagulating glands, and the levator ani without the bulbocavernosus muscle to a number of active chemicals, including estrogens and progesterones (Hershberger et al. 1953).
In the 1970s and 1980s, with the discovery of the androgen receptor and the first compounds such as cyprotone acetate that were antagonists of the receptor, the assay was modified to address antagonistic activity. Briefly, a set dose of a reference agonist was coadministered to several groups of animals that were also administered a set of doses of the purported antagonist. This modified system was successfully used by several investigators for assaying androgen antagonists (Peers et al. 1973; Raynaud et al. 1980, 1984; Wakeling et al. 1981).
Therefore, based upon the recommendation of scientific workshops, both the U.S. Endocrine Disruptor Screening and Testing Advisory Committee (U.S. EPA 1998) and the OECD Endocrine Disrupter Testing and Assessment Working Group (OECD 2000) have proposed this assay as a Tier 1 screen to identify possible reproductive and developmental toxicants acting through androgen agonist and antagonist mechanisms.
The OECD phase 1 validation program for the Hershberger assay was completed in 2001. In this phase, a standardized protocol using ventral prostate, seminal vesicles with coagulating glands, levator ani and bulbocavernosus muscle complex, Cowper's glands, and glans penis was successfully tested against a reference androgen compound, testosterone propionate (TP), and a reference antagonist, flutamide (OECD 2001). Therefore, the OECD proposed a phase 2 validation program using additional androgen agonists and antagonists as the next step to validate the assay.
In phase 2, the selected androgens were methyltestosterone (MT) and trenbolone; the selected antagonists were vinclozolin (VCZ), procymidone, linurone, and 2,2-bis (4-chlorophenyl)- 1,1-dichloroethylene (p,p'-DDE); and the 5[alpha]-reductase inhibitor was finasteride. These test substances will be used to investigate the reliability of the assay, including a demonstration of the protocol's transferability among laboratories and the reproducibility of the protocol's results. Seven Japanese laboratories participated in the phase 2 validation study that used three of the selected compounds: MT, VCZ, and p,p'-DDE. The participation of the laboratories in the OECD phase 2 validation study was performed as part of a national validation program in Japan.
Materials and Methods
Laboratories. The seven participating Japanese laboratories were the Chemicals Evaluation and Research Institute (CERI); the Food Drug Safety Center; the Institute of Environmental Toxicology; the Japan Bioassay Research Center; Mitsubishi Chemical Safety Institute; Panapham Co., Ltd.; and Sumitomo Chemical Company Ltd. Each laboratory performed in compliance with principles of good laboratory practice.
Test substances. The test substances were methyltestosterone (MT; CAS No. 58-18-4; 99.8% pure; Fluka Production GmbH, St. Louis, MO, USA), vinclozolin (VCZ; CAS No. 50471-44-8; 99% pure; Kanto Chemical Co., Tokyo, Japan), and p,p-DDE (CAS No. 72-55-9; 99.5% pure; Sigma-Aldrich Co., St. Louis, MO, USA). Testosterone propionate (TP; CAS No. 57-85-2; 97% pure; Fluka) was used as a reference positive chemical control and was coadministered with VCZ and p,p'-DDE to detect androgen antagonistic effects. MT, p,p'-DDE, and TP were obtained from a centralized chemical repository at TNO (Zeist, the Netherlands) and distributed through CERI to each laboratory; VCZ was obtained by CERI and distributed to each laboratory, in the study. All laboratories used corn oil as the vehicle. The test substances used in each laboratory, are shown in Table 1.
Animals. Laboratory details regarding rat strain, age at castration, number of postoperative acclimation days, age at autopsy, animal diet, and the number of animals housed per cage are summarized in Table 1. Five laboratories used Crj:CD (SD) (Sprague-Dawley) castrated rats from Charles River Japan, Inc. (Kanagawa/Shiga, Japan) between the ages of 40 and 46 days, and the test substances were administered 7-11 days after castration. Two laboratories used Brl Han: WIST Jcl (GALAS) castrated rats from Japan Clea, Inc. (Tokyo, Japan) between the ages of 40 and 43 days, and the test substances were administered 6 or 7 days after castration. In all of the laboratories, the rats were weighed, weight-ranked, and assigned to each of the experimental and control groups after they had recovered from castration. Body weight and clinical signs were recorded daily throughout the study. Rats were provided with water and a commercial diet (MF or CRF-1, Oriental Yeast Co., Tokyo, Japan) ad libitum. The animals were kept under specific-pathogen-free conditions. The animal room was maintained at a temperature of 23 [+ or -] 2[degrees]C, a relative humidity of 55 [+ or -] 15%, and artificial illumination with fluorescent light on a 12-hr light/dark cycle. All animals were cared for according to the principles outlined in the guide for animal experimentation prepared by the Japanese Association for Laboratory Animal Science (1992).
Chemical administration. Each rest chemical was orally administered via a stomach tube for 10 consecutive days at approximately the same time each day. A vehicle control group receiving only corn oil was used in all cases. For the androgen antagonists (VCZ and p,p'-DDE), 0.2 mg/kg/day of TP was coadministered each day by subcutaneous injection in the dorsal region after the oral administration of each chemical. In these cases, a positive control group of animals received TP injections alone. We selected the dose of TP on the basis of OECD recommendations and published data (OECD 2001; Sunami et al. 2000). The group size in all cases was six rats. For the TP and corn oil solutions containing each of the test chemicals, the volume of corn oil was 5 mL/kg. The MT doses were 0.05, 0.5, 5, and 50 mg/kg/day, and the VCZ and p,p'-DDE doses were 3, 10, 30, and 100 mg/kg/day. All doses were selected based on the results of preliminary, range-finding studies. The animals were killed by bleeding from the abdominal vein under deep ether anesthesia approximately 24 hr after receiving their final dose. The five mandatory tissues--the ventral prostate and fluid, seminal vesicle and fluid, bulbocavernosus/levator ani muscle (BC/LA), glans penis, and Cowper's gland--were carefully dissected free of adhering fat and weighed to the nearest 0.1 mg. Six of the laboratories weighed the wet organs. One laboratory (Lab 4) weighed the prostate, seminal vesicle, Cowper's glands, and adrenal glands after approximately 24 hr fixation in 10% formalin solution, following the procedure of Yamada et al. (2000). The liver, paired kidneys, and paired adrenal glands were weighed as optional organs in some laboratories in each assay described in Table 2.
Statistical analysis. Body weight and organ weight data were tested using Bartlett's test for homogeneity of variance. When the variances were homogeneous at the 5% significance level, one-way analysis of variance (ANOVA) was performed. If it yielded significant differences, the differences between the vehicle control group and each of the MT groups or the positive control group and each of the VCZ and p,p'-DDE groups were analyzed by Dunnett's test. When the variances were nor homogeneous, the Kruskal-Wallis test was used. If it yielded significant differences, the differences between each group and the corresponding control group were analyzed by the nonparametric Dunnett's test. Log-transformed organ-weight data were also tested by the same method. The coefficient of variance (CV) and [R.sup.2] values for the different effects of each compound were also calculated by dividing the sums of the squares of the ANOVA scores for an effect by the total sum of the squares. This calculation provides an estimate of the strength of an effects association with an end point. Data for each end point were also analyzed using a two-way ANOVA, with dosage and laboratory as the main effects, so that the magnitude of the overall dosage and laboratory effects could be determined. For graphic presentation, the sex accessory organ data were normalized to visually compare the shapes of the dose response curves produced by each laboratory. For this normalization, the control value was set to 100% in the MT assay, and 100% in the TP without VCZ or p,p'-DDE assays. ANOVA was performed on the data from each laboratory and for the pooled laboratory data; these normalized values were not analyzed statistically,
Results
Methyltestosterone. Body weights, clinical observations, and organ weights. The weight changes in optional organs and the body weights on the first day of dosing and at necropsy are shown in Tables 2 and 3. No significant differences in body weight were observed between the vehicle control group and the MT group in each laboratory. No abnormal clinical signs were observed in any of the rats that were treated with MT. The paired kidney weights increased significantly at 50 mg/kg/day MT in Lab 4, and adrenal weights decreased at the same dose in Lab 4.
Accessory sex organ weights. Accessory sex organ weight changes and overall means are shown in Tables 3 and 4, and normalized organ weight changes are shown in Figure 1.
[FIGURE 1 OMITTED]
For the ventral prostate, the normalized dose-response curves produced by the four laboratories were similar, and the weight change at 50 mg/kg/day MT relative to the vehicle control ranged from 641% to 1,022%. This was the largest weight change observed in any of the examined organs. The [R.sup.2] values for effects of treatments (TRT) in the ventral prostate was higher than the respective TRT values for other organs.
The normalized dose response curves produced by the four laboratories were similar for the seminal vesicle; the weight change ranged from 465% to 707% at 50 mg/kg/day MT relative to the vehicle control.
For BC/LA, the normalized dose-response curves produced by the four laboratories were almost the same, and the weight change at 50 mg/kg/day MT relative to the vehicle control ranged from 226% to 240%.
The normalized dose-response curves produced by the four laboratories were similar for the glans penis, and the weight change at 50 mg/kg/day MT relative to the vehicle control ranged from 150% to 162%. Although the range between the low and high relative weight changes in animals receiving 50 mg/kg/day MT was narrow, the relative weight increase at this dose was the smallest of the weight changes in all of the accessory sex organs that were examined. The average CV for the glans penis was the lowest of all the average values obtained for the other organs. The [R.sup.2] values for effects among laboratories (LAB) for the glans penis was the highest value obtained among the accessory sex organs examined in this study.
For the Cowper's glands, the normalized dose-response curves produced by the four laboratories were similar, and the weight change ranged from 273% to 417% at 50 mg/kg/day MT relative to the vehicle control.
Vinclozolin. Body weights, clinical general observations, and organ weights. The weight changes in optional organs and the body weight changes for VCZ-treated rats are shown in Tables 2 and 5. No significant differences in body weight were observed between the positive control group that received TP injections alone and the VCZ group in any of the laboratories. No abnormal clinical signs were observed in any of the rats treated with VCZ plus TP. Weight of the paired adrenal glands increased significantly at 100 mg/kg/day, and no other significant changes were detected in the liver and paired kidneys.
Accessory sex organ weights. Weight changes in accessory sex organs and overall means are shown in Tables 5 and 6, and normalized organ weight changes are shown in Figure 2.
[FIGURE 2 OMITTED]
For the ventral prostate, the normalized dose-response curves produced by the four laboratories were similar. The ventral prostate weight changes at 100 mg/kg/day VCZ relative to the positive control ranged from 27% to 37%.
The normalized dose-response curves produced by the four laboratories were similar for seminal vesicles. The weight changes at 100 mg/kg/day VCZ relative to the positive control were similar, ranging from 15% to 23%. These values were the lowest of all the values for the accessory sex organs, and the decreasing dose-response curve for the seminal vesicle was sharper than the curves for the other organs.
For the BC/LA, the normalized dose-response curves produced by the four laboratories were similar, and the weight change at 100 mg/kg/day VCZ relative to the positive control were similar, ranging from 48% to 52%.
The normalized dose-response curves produced by the four laboratories were similar for the glans penis, and the weight change at 100 mg/kg/day VCZ relative to the positive control were similar, ranging from 69% to 73%. The overall CV value was the lowest among the values for the examined accessory sex organs.
The normalized dose-response curves produced by the four laboratories were similar for Cowper's glands. The weight change ranged from 36% to 42% at a dose of 100 mg/kg/day VCZ relative to the positive control.
p,p'-DDE. Body weights, clinical observations, and organ weights. The weight changes in optional organs and the body weight changes for p,p'-DDE-treated rats are shown in Tables 2 and 7. The body weight decreased significantly in the 100 mg/kg/day group of lab 5, and a similar (but not significant) tendency was also observed in the 100 mg/kg/day group of Lab 2. No abnormal clinical signs were detected in any of the rats treated with p,p'-DDE plus TP. The liver weights increased significantly at 30 and 100 mg/kg/day in Lab 4. No significant changes were observed in other organs.
Accessory sex organ weights. Weight changes in accessory sex organs and overall means are shown in Tables 7 and 8, and normalized organ weight changes are shown in Figure 3.
[FIGURE 3 OMITTED]
For the ventral prostate, the normalized dose-response curves produced by the five laboratories were very similar, except for the curve produced by Lab 7 because of the value at 30 mg/kg/day p,p'-DDE. The weight change at a dose of 100 mg/kg/day relative to the positive control ranged from 37% to 62%.
The normalized dose-response curves produced by the laboratories were similar at 10, 30, and 100 mg/kg/day p,p'-DDE for seminal vesicle. The weight change of the seminal vesicles at 100 mg/kg/day relative to the positive control ranged front 23% to 54%. The dose-response curve for the seminal vesicle was the sharpest of the various curves produced for the accessory sex organs for p,p'-DDE. The TRT in the seminal vesicle was the highest value among the accessory sex organs measured in this study.
For BC/LA, the normalized dose-response curves produced by four laboratories were similar. The weight change ranged from 55% to 72% at 100 mg/kg/day p,p'-DDE relative to the positive control.
The normalized dose-response curves were similar in glans penis above a dose of 30 mg/kg/day p,p'-DDE. The weight change at 100 mg/kg/day p,p'-DDE relative to the positive control ranged from 79% to 86%, and this percentage was the highest among the values for the accessory sex organs receiving p,p'-DDE. The CV of the glans penis and the BC/LA were smaller than the values for the other organs. The TRT for the glans penis was the smallest of the values observed among the accessory sex organs in p,p'-DDE-treated rats.
For Cowper's glands, the normalized dose-response curves produced by the laboratories were similar above a dose of 30 mg/kg/day p,p'-DDE. The weight change at 100 mg/kg/day relative to the positive control ranged from 41% to 65%.
Discussion
Seven Japanese laboratories performed the Hershberger assay using MT, VCZ, and p,p'-DDE as part of a national validation program. The weights of all the accessory sex organs from the experimental animals in all the laboratories exhibited significant dose-related changes in the assays using agonistic MT or antagonistic VCZ and p,p'-DDE; the normalized dose-response curves showed that all five tissues reacted in a similar manner for each compound. Furthermore, the weights of all the tissues treated with middle and/or high doses in each assay fell within narrow ranges. Therefore, we consider the Hershberger assay, as proposed by the OECD, to be a good screening assay for detecting the androgen agonistic and antagonistic effects of chemicals.
The OECD proposed TP doses of 0.2 mg/kg/day and 0.4 mg/kg/day to detect antagonistic effects of chemicals based on the data from the OECD phase 1 validation of the Hershberger assay (OECD 2001). In the previous study, we used the 0.2 mg/kg/day dose of TP in Hershberger assays of 30 chemicals based on the OECD draft protocol and found that the accessory sex organ weights of the castrated rats were lower than those of castrated rats given TP, and the weights of these organs in rats given 10 mg/kg/day flutamide plus TP were also lower than in castrated rats given TP (Yamasaki et al. 2003). In addition, the weights of the accessory sex organs of the castrated rats were lower than those of castrated rats given 0.4 mg/kg/day TP, and their weights were also lower in noncastrated rats than in castrated rats given TP (Yamasaki et al. 2002). We selected the 0.2 mg/kg/day dose in this study, however, a dose of 0.4 mg/kg/day was used in the phase 2 validation studies except in Japan (OECD 2003). The sensitivity of this assay of antagonistic chemicals at the 0.2 mg/kg/day and 0.4 mg/kg/day doses needs to be compared.
The OECD phase 1 validation of the Hershberger assay using antagonistic flutamide reported that the seminal vesicle exhibited the most sensitive end point and that the glans penis exhibited the least sensitive end point, based on benchmark dose estimates (OECD 2001). When the overall dose-response curves for agonistic TP were compared, the glans penis was the most sensitive and the seminal vesicle was the least sensitive (OECD 2001). In the present study, it was difficult to select a particularly sensitive organ from among the five tissues examined in the androgen agonistic MT and antagonistic VCZ and p,p'-DDE assays. In the Hershberger assay using MT, the CV for the glans penis was smaller than that of the other organs, but the TRT of the ventral prostate was the highest among the values measured in the study. On the other hand, the LAB values of the ventral prostate and Cowper's glands were smaller than the values of the other organs, and the percentage weight change relative to the control value at the highest dose was the greatest in the ventral prostate. These findings demonstrate that the ventral prostate was particularly sensitive based on the TRT, LAB, and increasing percentage of organ weight, whereas the glans penis was sensitive based on the CV values. Similarly, the seminal vesicle was sensitive based oil the TRT, LAB, and decreasing percentage of organ weight, whereas the glans penis was sensitive based on the CV values in the assays using antagonistic VCZ and p,p'-DDE.
The CV values for the ventral prostate, seminal vesicle, and Cowper's gland were higher than those for the glans penis and BC/LA in the assays for all three chemicals. These organs contain fluid, and the dissection of these organs is technically difficult, compared with that of the glans penis and BC/LA. These technical issues may have influenced the varied CV values obtained for these organs. Furthermore, we did not confirm whether preputial separation had occurred in the rats before castration. Preputial separation has been reported to occur between days 39 and 44 in SD rats (Yamasaki et al 2001); in this study, the castration was performed between days 40 and 46. Thus, the rats used in this study were likely a mixture of animals with or without preputial separation. The castration times may also have influenced the variation in the CV values for each organ.
In the assay using the androgen antagonistic chemicals, slight differences in the normalized response curves for low doses in the p,p'-DDE assay were observed among the laboratories, but the response curves for each organ in the VCZ assay were similar. The fact that the percentages of organ weight relative to the control at high doses in the p,p'-DDE assay were lower than those in the VCZ assay suggests that the androgen antagonistic affinity of p,p'-DDE is weaker than that of VCZ. On the other hand, the organ weights of the rats given only TP varied among the laboratories. The slight variation in responses among the laboratories for the low dose in the p,p'-DDE assay may have been affected by the relationship between the agonistic affinity of TP and the weak antagonistic affinity of p,p'-DDE.
In the phase 1 validation study using TP, the OECD reported that no essential differences were observed when the weights of the fresh and fixed organs were compared (OECD 2001). Lab 4 weighed the prostate, seminal vesicle, and Cowper's glands after fixation, whereas the other laboratories measured the weights of fresh organs; the changes in organ weight among the laboratories were essentially similar. Therefore, the difference in the weighing method (fresh vs. fixed organs) did not appear to affect the results of the assay. Although the terminal body weights were different between SD and Wistar rats, the responsiveness of these rats to VCZ and p,p'-DDE did not differ in this study. This finding demonstrates that no significant differences exist regarding the use of SD and Wistar rats in the Hershberger assay for the detection of androgen antagonists.
Among the optional organs measured in this study, the weight of the adrenal glands increased significantly in rats given 100 mg/kg/day of VCZ and decreased in rats given 50 mg/kg/day MT. The decrease in adrenal weight may be suppressed by a high dose of androgen in the form of MT, and the adrenal glands may be hypertrophied in response to a high level of antagonist. Increased kidney weights in rats given 50 mg/kg/day of MT and increased liver weights in rats given 30 and 100 mg/kg/day of p,p'-DDE suggested toxic effects. On the other hand, a significant decrease or a tendency to decrease of the body weights in the p,p'-DDE assay was observed by two out of five laboratories; this response was also considered to be a toxic effect of p,p'-DDE.
REFERENCES
Bulbring E, Burn JH. 1935. The estimation of oestrin and of male hormone in oily solution. J Physiol 85:320-333.
Deanesly R, Parkes AS. 1936. Comparative activities of compounds of the androsterone-testosterone series. Biochem J 30:291-303.
Dingemanse E, Frued J, Laquer E. 1935. Differences between male hormone extracts from urine and from testes. Nature 135:184.
Di Salle E, Briatico G, Giudici D, Ornati G, Panzeri A. 1994 Endocrine properties of the testosterone 5[alpha]-reductase inhibitor turosteride (FCE 26073). J Steroid Biochem Mol Biol 48:241-248.
Eisenberg E, Gordan GS. 1950. The levator ani muscle of the rat as an index of mytrophic activity of steroidal hormones. J Pharmacol Exp Therap 99:38-4.
Eisenberg E, Gordan GS, Elliott HW. 1949 Testosterone and tissue respiration of the castrate male rat with a possible test for mytrophic activity. Endocrincology 45:113-119.
Hershberger LG, Shipley EG, Meyer RK. 1953. Myotrophic activity of 19-nortestosterone and other steroids determined by modified levator ani muscle method. Proc Soc Exp Biol Med 83:175-80.
Japanese Association for Laboratory Science. 1992. The Guidelines for Animal Experimentation Tokyo:Soft Science Inc.
Korenchevsky V. 1932. The assay of testicular hormone preparations. Biochem J 26:413-422.
Korenchevsky V, Dennison M, Kohn-Speyer A. 1933a. Changes produced by testicular hormone in normal and in castrated rats. Biochem J 27:557-579.
--. 1933b. On the assay and the absorption of testicular hormone dissolved in oil. Biochem J 27:778-782.
Korenchevsky V, Dennison M, Schalit R. 1932. The response of castrated male rats to the injection of the testicular hormone. Biochem J 26:1306-1314.
McLachlan JA. 1993. Functional toxicology: a new approach to detect functionally active xenobiotics. Environ Health Perspect 101:380-387.
McLachlan JA, Korach KS. 1995. Symposium on estrogens in the environment, III. Environ Health Perspect 103(suppl 7):3-4.
OECD. 1998. Report of the First Meeting of the OECD Endocrine Disrupter Testing and Assessment (EDTA) Working Group. Paris:Organisation for Economic Co-operation and Development
--. 2000. The Second Meeting of the OECD Validation Management Group (VMG) for the Screening and Testing of Endocrine Disrupters. Paris:Organisation for Economic Co-operation and Development.
--. 2001. Third Meeting of the OECD Validation Management Group (VMG) for the Screening and Testing of Endocrine Disrupters. Paris:Organisation for Economic Co-operation and Development.
--. 2003. Fourth Meeting of the OECD Validation Management Group (VMG) for the Screening and Testing of Endocrine Disrupters. Paris:Organisation for Economic Co-operation and Development.
Peets EA, Henson MF, Neri R. 1973. On the mechanism of the antiandrogenic action of flutamide ([alpha]-[alpha]-[alpha]-trifluoro-2-methyl-4'-nitro-m-propionotoluidide) in the rat. Endocrinology 94:532-540.
Raynaud JP, Bonne C, Moguilewsky M, Lefebvre FA, Belanger A, Labrie F. 1984. The pure antiandrogen RU 23908 (Anandron), a candidate of choice for the combined anti-hormonal treatment of prostatic cancer: a review. Prostate 5:299-311.
Raynaud JP, Bouton MM, Moguilewsky M, Ojasoo T, Philibert D, Beck G, et al. 1980. Steroid hormone receptors and pharmacology. J Steroid Biochem 12:143-157.
Sunami O, Kunimatsu T, Yamada T, Yabushita S, Sukata T, Miyata K, et al. 2000. Evaluation of a 5-day Hershberger assay using young mature male rats: methyltestosterone and p,p'-DDE, but not fenitrothion, exhibited androgenic or antiandrogenic activity in vivo. Toxicol Sci 25:403-415.
U.S. Environmental Protection Agency. 1998. Endocrine Disruptor Screening and Testing Advisory Committee (EDSTAC) Final Report. EPA/743/R-98/003. Available: http://www.epa.gov/ scipoly/oscpendo/history/finalrpt.htm [accessed 27 October 2003].
Wainman P, Shipounoff GC. 1941. The effects of castration and testosterone propionate on the striated perineal musculature in the rat. Endocrinology 29:975-978.
Wakeling A, Furr BJA, Glen AT, Hughes LR. 1981. Receptor binding and biological activity of steroidal and nonsteroidal antiandrogens. J Steroid Biochem 15:355-359.
Yamada T, Sunami O, Kunimatsu T, Kamita Y, Okuno Y, Seki T, et al. 2000. Dissection and weighing of accessory sex glands after formalin fixation, and a 5-day assay using young mature rats are reliable and feasible in the Hershberger assay. Toxicology 162:103-119.
Yamasaki K, Sawaki M, Noda S, Muroi T, Takatsuki M. 2001. Preputial separation and glans penis changes in normal growing Crj:CD(SD) IGS rats. Reprod Toxicol 15:533-535.
Yamasaki K, Sawaki M, Noda S, Takatsuki M. 2002. Uterotrophic and Hershberger assays for n-butylbenzene in rats. Arch Toxicol 75:703-706.
Yamasaki K, Takeyoshi M, Yakabe Y, Sawaki M, Imatanaka M, Shinoda K, et al. 2003. Immature rat uterotrophic assay of 18 chemicals and Hershberger assay of 30 chemicals. Toxicology 183:95-115.
Kanji Yamasaki, (1) Masakuni Sawaki, (1) Ryo Ohta, (2) Hirokazu Okuda, (3) Seiichi Katayama, (4) Tomoya Yamada, (5) Takafumi Ohta, (6) Tadashi Kosaka, (7) and William Owens (8)
(1) Chemicals Evaluation and Research Institute, Oita, Japan; (2) Food Drug Safety Center, Kanagawa, Japan; (3) Japan Bioassay Research Center, Kanagawa, Japan; (4) Mitsubishi Chemical Safety Institute, Ibaraki, Japan; (5) Sumitomo Chemical Company, Osaka, Japan; (6) Panapham Laboratories Co., Ltd., Kumamoto, Japan; (7) Institute of Environmental Toxicology, Ibaraki, Japan; (8) Environmental Health and Safety Division, Organisation for Economic Co-operation and Development, Paris, France
Address correspondence to K. Yamasaki, Chemicals Assessment Center, Chemicals Evaluation and Research Institute, 3-822, Ishii, Hita, Oita 087-0061, Japan. Telephone: 81-973-24-7211. Fax: 81-97323-9800. E-mail: yamasaki-kanji@ceri.jp
This study was supported by grants from the Ministry of Economy, Trade, and Industry; the Ministry of Health, Labour, and Welfare; and the Ministry, of the Environment in Japan.
The authors declare they have no competing financial interests.
Received 27 March 2003; accepted 10 September 2003.
COPYRIGHT 2003 National Institute of Environmental Health Sciences
COPYRIGHT 2004 Gale Group