METHOD OF PREPARATION
1. Calculate the required quantity of each ingredient for the total amount to be prepared.
2. Accurately weigh and/or measure each ingredient.
3. Mix the misoprostol and the triamcinolone acetonide with the glycerin to form a smooth paste.
4. Dissolve the diphenhydramine hydrochloride, lidocaine hydrochloride and potassium sorbate in about 60 mL of purified water.
5. Add the sorbitol 70% solution to the misoprostol-triamcinolone-glycerin mixture and mix until uniform.
6. Add the aqueous solution of the water soluble drugs and the flavor and mix well.
7. Adjust the pH with the citric acid solution to the range of 5.5 to 6.0.
8. Add sufficient purified water to volume and mix well.
9. Package and label.
PACKAGING
Package in tight, light-resistant containers.1
LABELING
Keep out of reach of children. Use only as directed. Shake well before using. Keep in refrigerator.
STABILITY
A beyond-use date of 14 days would be appropriate for this preparation when stored in a refrigerator.1
USE
This preparation has been used in the treatment of oral mucositis.
QUALITY CONTROL
Quality-control assessment can include weight/volume, pH, active drug assay, color, physical observation and physical stability.2
DISCUSSION
There are numerous combinations of misoprostol and lidocaine with different corticosteroids and antihistamines that are commonly used for the treatment of oral mucositis or for the prevention of radiation-induced oral mucositis.
Diphenhydramine hydrochloride (C^sub 17^H^sub 21^NO.HCl, MW 291.82) occurs as a white, odorless, crystalline powder. It is freely soluble in water and in alcohol. It is a Hi-receptor antagonist used for the relief of hypersensitivity reactions, nausea, vomiting and vertigo.1
Lidocaine hydrochloride (C^sub 14^H^sub 22^N^sub 2^O-HCl.H^sub 2^O, MW 288.81) occurs as a white, odorless, crystalline powder with a slightly bitter taste. It is an amide-type local anesthetic with a rapid onset and intermediate duration of action. It is very soluble in water (1:0.7) and in alcohol (1:1.5).1,3
Misoprostol (C^sub 22^H^sub 38^O^sub 5^, MW 382.53, Cytotec) is a synthetic analog of prostaglandin EI (alprostadil) and occurs as a watersoluble, viscous liquid. It is a gastric antisecretory agent with protective effects on the gastroduodenal mucosa. Cytotec tablets contain either 100 µg or 200 µg of misoprostol. The tablets also contain hydrogenated castor oil, hydroxypropyl methylcellulose, microcrystalline cellulose and sodium starch glycolate.4
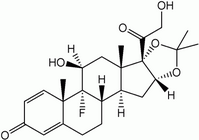
Triamcinolone acetonide (C^sub 24^H^sub 31^FO^sub 6^, MW 434.51) occurs as a white-to-cream colored, crystalline powder with not more than a slight odor. It is practically insoluble in water and sparingly soluble in dehydrated alcohol.1
Glycerin (C^sub 3^H^sub 8^O^sub 3^, MW 92.10, glycerol, 1,2,3-propane triol) occurs as a clear, colorless, odorless, viscous, hygroscopic liquid with a sweet taste about two thirds as sweet as sucrose. It is miscible with water, methanol and 95% ethanol, practically insoluble in oils and slightly soluble in acetone.5
Sorbitol (C^sub 6^H^sub 14^O^sub 6^, MW 182.17, D-glucitol) is a hexahydric alcohol related to mamiose and is isomeric with mannitol. It occurs as an odorless, white or almost colorless, crystalline, hygroscopic powder. It has a pleasant, cooling, sweet taste.1
Potassium sorbate (C^sub 6^H^sub 7^KO^sub 2^, MW 150.22) occurs as white crystals or powder with a characteristic odor. It is freely soluble in water and soluble in alcohol. It is an antimicrobial preservative.1
Citric acid (C^sub 6^H^sub 8^O^sub 7^.H^sub 2^O, MW 210.14, citric acid monohydrate) occurs as colorless or translucent crystals or as a white crystalline, efflorescent powder that is odorless and has a strong tart, acidic taste. One gram is soluble in less than 1 mL of water and 1.5 mL of ethanol.7
REFERENCES
1. US Pharmacopeial Convention, Inc. United States Pharmacopeia 27National Formulary22. Rockville, MD: US Pharmacopeial Convention, Inc.; 2004: 1087-1088, 1875-1876, 2267, 2345-2349, 2768, 2778, 2921.
2. Alien LV Jr. Standard operating procedure for performing physical quality assessment of oral and topical liquids. IJPC1999; 3(2): 146-147.
3. McEvoy GK. AHFS Drug Information-2004. Bethesda, MD: American Society of Health-System Pharmacists; 2004: 2807-2812, 3089-3090.
4. [No author listed.] Physicians' Desk Reference. 58th ed. Montvale, NJ: Thomson PDR; 2004: 3109-3111.
5. Price JC. Glycerin. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC: American Pharmaceutical Association; 2003: 257-259.
6. Nash RA. Sorbitol. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC: American Pharmaceutical Association; 2003: 596-599.
7. Amidon GE. Citric acid monohydrate. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC: American Pharmaceutical Association; 2003: 158-160.
Copyright International Journal of Pharmaceutical Compounding Jul/Aug 2004
Provided by ProQuest Information and Learning Company. All rights Reserved