METHOD OF PREPARATION
1. Calculate the required quantity of each ingredient for the total amount to be prepared.
2. Accurately weigh and/or measure each ingredient.
3. Mix the perphenazine with the glycerin.
4. Incorporate into the Ora-Sweet or the Ora-Sweet SF and mix well.
5. Package and label.
PACKAGING
Package in tight, light-resistant containers.1
LABELING
Shake well. Keep out of reach of children. Use only as directed.
STABILITY
A beyond-use date of up to 6 months should be reasonable for this preparation.1
USE
Perphenazine syrup is used in the management of the manifestations of psychotic disorders and for the control of severe nausea and vomiting in adults.2
QUALITY CONTROL
Quality-control assessment can include weight/volume, pH, specific gravity, active-drug assay, color, clarity, physical observation and physical stability (discoloration, foreign materials, gas formation, mold growth).3
DISCUSSION
Perphenazine has pharmacologie effects similar to those of chlorpromazine. It has moderate anticholinergic effects, weak-to-moderate sedative effects and strong extrapyramidal effects. It also has strong antiemetic activity. Perphenazine 2.5-mg/5-mL syrup was formerly commercially available as Trilafon 2.5-mg/5-mL Syrup. Trilafon preparations still commercially available include Trilafon Concentrate 16 mg/5 mL, Trilafon Tablets (2, 4, 8, and 16 mg), and Trilafon 5-mg/mL Injection. It is also available in combination with amitriptyline hydrochloride.2
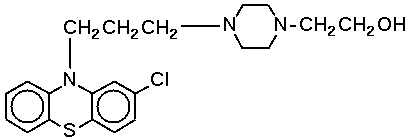
Perphenazine (C^sub 21^H^sub 26^ClN^sub 3^OS, MW 403.97) is a phenothiazine antipsychotic agent that is a propylpiperazine derivative of phenothiazine. It occurs as a white to creamy-white, odorless powder with a bitter taste. It is practically insoluble in water and is freely soluble in alcohol (153 mg/mL). It is practically insoluble in sesame oil. It has a melting point of 214 to 218°C and is sensitive to light. The commercially available oral concentrate solution has a pH in the range of 4.5 to 4.9.1,4
Glycerin (C^sub 3^H^sub 8^O^sub 3^, MW 92.1, glycerol, 1,2,3-propane triol) occurs as a clear, colorless, odorless, viscous, hygroscopic liquid with a sweet taste about two thirds as sweet as that of sucrose. It is used as an antimicrobial preservative (>20% concentration), an emollient and humectant (up to 30% concentration), in ophthalmic formulations (0.5% to 3% concentration), as a plasticizer in film coating for tablets, as a parenteral solvent (up to 50% concentration) and as a sweetening agent in alcoholic elixirs (up to 20% concentration). It has a specific gravity of about 1.25 and a melting point of 17.8°C; if cooled to crystallization, it will need to be heated to about 20°C to melt. It is miscible with water, methanol and 95% ethanol; practically insoluble in oils and chloroform; and slightly soluble in acetone. It is hygroscopic and should be stored in airtight containers in a cool place. It is not prone to oxidation but will decompose on heating. When mixed with water, ethanol and propylene glycol, the mixtures are chemically stable.5
Ora-Sweet syrup vehicle is a flavoring vehicle for oral extemporaneous preparations. It is flavored with a citrus-berry flavor blend and contains glycerin and sorbitol to prevent "cap-lock," a problem associated with many syrups. It is buffered to a pH of approximately 4.2 and has an osmolality of about 3240 mOsm/Kg. It contains purified water, sucrose, glycerin, sorbitol (5%), flavoring, sodium phosphate and citric acid as buffering agents, and potassium sorbate and methylparaben as preservatives.6
Ora-Sweet SF is a flavoring vehicle for oral extemporaneous preparations. It is a sugar-free, alcohol-free syrup flavored with a citrus-berry flavor blend. It is buffered to a pH of approximately 4.2 and may be used alone or in combination with other vehicles. It will tolerate a dilution to 50% with dissolved actives in water or suspending agents and still retain an acceptable taste. It has an osmolality of 2150 mOsm/Kg. It contains water, sodium saccharin, xanthan gum, glycerin, sorbitol, citric acid and sodium citrate as buffers; methylparaben, propylparaben and potassium sorbate as preservatives; and flavoring-agents.6
REFERENCES
1. US Pharmacopeial Convention, Inc. United States Pharmacopeia 26-National Formulary 21. Rockville, MD:US Pharmacopeial Convention, Inc; 2003:2197-2201, 2574.
2. McEvoy GK. AHFS Drug Information-2003. Bethesda, MD:American Society of Health-System Pharmacists; 2003:2267-2269.
3. Allen LV Jr. Standard operating procedure for performing physical quality assessment of oral and topical liquids. IJPC 1999;3:146-147.
4. [No author listed.] Merck Index. 13th ed. Whitehouse Station, NJ:Merck & Co., Inc.; 2001:7267.
5. Price JC. Glycerin. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Washington, DC:American Pharmaceutical Association; 2003:257-259.
6. Ora-Sweet [product information]. Minneapolis, MN:Paddock Laboratories, Inc.
Copyright International Journal of Pharmaceutical Compounding Nov/Dec 2003
Provided by ProQuest Information and Learning Company. All rights Reserved