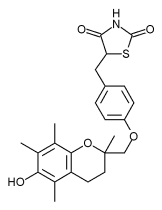
An FDA advisory panel has recommended that troglitazone (Rezulin) be used only as a second-line diabetes therapy in combination with other diabetes drugs (F-D-C Reports. 1999;March 29:14-15). A majority of the panelists voted to recommend that the benefits of troglitazone as monotherapy do not outweigh the risks associated with use of the drug. Panel members further recommended that troglitazone's approved indications be changed so that the drug may only be used in combinations to treat patients who have failed other therapies.
Advisory panel members want comprehensive patient education materials to be given to patients taking troglitazone, hoping that such material will improve compliance with required monthly liver enzyme tests. Furthermore, patient education materials may help alert patients to the signs of liver problems so they can seek early medical attention.
Copyright Springhouse Corporation Jun 1999
Provided by ProQuest Information and Learning Company. All rights Reserved