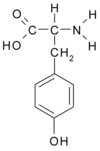
ABSTRACT Tyrosine ring dynamics of the gastrointestinal hormone motilin was studied using two independent physical methods: fluorescence polarization anisotropy decay and NMR relaxation. Motilin, a 22-residue peptide, was selectively '3C labeled in the ring E-carbons of the single tyrosine residue. To eliminate effects of differences in peptide concentration, the same motilin sample was used in both experiments. NMR relaxation rates of the tyrosine ring C^sup epsilon^-H^sup epsilon^ vectors, measured at four magnetic field strengths (9.4,11.7,14.1, and 18.8 Tesla) were used to map the spectral density function. When the data were analyzed using dynamic models with the same number of components, the dynamic parameters from NMR and fluorescence are in excellent agreement. However, the estimated rotational correlation times depend on the choice of dynamic model. The correlation times estimated from the two-component model-free approach and the three-component models were significantly different (1.7 ns and 2.2 ns, respectively). Various earlier studies of protein dynamics by NMR and fluorescence were compared. The rotational correlation times estimated by NMR for samples with high protein concentration were on average 18% longer for folded monomeric proteins than the corresponding times estimated by fluorescence polarization anisotropy decay, after correction for differences in viscosity due to temperature and D^sub 2^O/H^sub 2^O ratio.
INTRODUCTION
NMR relaxation and fluorescence polarization anisotropy decay (FAD) are two important experimental methods to study the dynamics of biomolecules. The results from the two methods on protein dynamics have been compared for a number of proteins listed in Table 1. In several cases the global rotational correlation time deviates significantly between the two methods. In most cases the correlation time observed by fluorescence is shorter than the correlation time observed by NMR. The details of this table will be discussed later. A fraction of the observed discrepancies between the NMR data and the fluorescence data can be explained by the typical difference in concentration between NMR and fluorescence studies.
Because FAD and NMR relaxation are about the only two experimental approaches to detailed studies of molecular dynamics, it is important to try to reconcile the results and find out where the results from the two methods deviate from each other and become less reliable.
Motilin is a gastrointestinal peptide hormone with 22 amino acids, among them the single Tyr^sup 7^ fluorophore. In our previous study of motilin dynamics (Allard et al., 1995; Jarvet et al., 1996) we found that the overall rotational correlation time of motilin is ~5 ns at 20 deg C and 3 ns at 35 deg C in 30% hexafluoro-2-propanol (HFP), evaluated by spectral density mapping and with Leuo a-carbon as the probe for the relaxation measurements. These results were significantly different from the value (2.2 ns at 20 deg C in 30% HFP) measured by FAD on the single Tyr residue of the peptide.
We thank Britt-Marie Olsson for peptide synthesis and Kalle Kaljuste for help with attaching the t-Boc protecting group on the labeled tyrosine. We acknowledge the Swedish NMR center for the use of their 500- and 800-MHz NMR spectrometers.
This work was supported by a grant from the Swedish Research Council.
Submitted May 19, 2002, and accepted for publication June 5, 2002.
REFERENCES
Akke, M., N. J. Skelton, J. Kordel, A. G. Palmer, and W. J. Chazin. 1993. Effects of ion-binding on the backbone dynamics of calbindin-D9k determined by 15N NMR relaxation. Biochemistry. 32:9832-9844.
Allard, P., M. Helgstrand, and T. Hard. 1998. The complete homogeneous master equation for a heteronuclear two-spin system in the basis of Cartesian product operators. J. Magn. Reson. 134:7-16.
Allard, P., J. Jarvet, A. Ehrenberg, and A. Graslund. 1995. Mapping of the spectral density function of a Ca-H' bond vector from NMR relaxation rates of a13C-labelled a-carbon in motilin. J. BiomoL NMR. 5:133-146.
Backlund, B. M., T. Kulinski, R. Rigler, and A. Graslund. 1995. Dynamics of the peptide hormone motilin studied by time resolved fluorescence spectroscopy. Eur. Biophys. J. 23:407-412.
Bonincontro, A., V. Calandrini, and G. Onori. 2001. Rotational and translational dynamics of lysozyme in water-glycerol solution. Colloids Surf. B Biointerfaces. 21:311-316.
Bremi, T., R. Bruschweiler, and R. R. Ernst. 1997. A protocol for the interpretation of side-chain dynamics based on NMR relaxation: application to phenylalanines in antamanide. J. Am. Chem Soc. 119:4272-4284.
Buck, M., J. Boyd, C. Redfield, D. A. MacKenzie, D. J. Jeenes, D. B. Archer, and C. M. Dobson. 1995. Structural determinants of protein dynamics: analysis of tSN NMR relaxation measurements for main-chain and sidechain nuclei of hen egg white lysozyme. Biochemistry. 34:4041-4055.
Case, D. A. 1999. Calculations of NMR Bipolar coupling strengths in model peptides. J. Biomol. NMR. 15:95-102.
Chen, L. X. Q., J. W. Longworth, and G. R. Fleming. 1987. Picosecond time-resolved fluorescence of ribonuclease-T1: a pH and substrate-- analog binding study. Biophys. J. 51:865-873.
Chirico, G., S. Beretta, and G. Baldini. 1999. Conformation of interacting lysozyme by polarized and depolarized light scattering. J. Chem. Phys. 110:2297-2304.
Clore, G. M., A. Szabo, A. Bax, L. E. Kay, P. C. Driscoll, and A. M. Gronenborn. 1990. Deviations from the simple 2-parameter model-free approach to the interpretation of N-15 nuclear magnetic-relaxation of proteins. J. Am. Chem. Soc. 112:4989-4991.
Damberg, P., J. Jarvet, P. Allard, and A. Graslund. 1999. Quantitative estimation of magnitude and orientation of the CSA tensor from field dependence of longitudinal NMR relaxation rates. J. Biomol. NMR; 15:27-37.
Damberg, P., J. Jarvet, and A. Graslund. 2001. Accurate measurement of translational diffusion coefficients: a practical method to account for nonlinear gradients. J. Magn. Reson. 148:343-348.
Dayie, K. T., and G. Wagner. 1994. Relaxation-rate measurements for ESN-1H groups with pulsed-field gradients and preservation of coherence pathways. J. Magn. Reson. Ser. A. 111:121-126.
De Paul, S. M., K. Saalwachter, R. Graf, and H. W. Spiess. 2000. Sideband patterns from rotor-encoded longitudinal magnetization in MAS recoupling experiments. J. Magn. Reson. 146:140-156.
Dill, K., and A. Allerhand. 1979. Small errors in C-H bond lengths may cause large errors in rotational correlation times determined from carbon-13 spin-lattice relaxation measurements. J Am. Chem Soc. 101:4376-4378.
Farrow, N. A., 0. Zhang, A. Szabo, D. A. Torchia, and L. E. Kay. 1995. Spectral density function mapping using ISN relaxation data exclusively. J. Biomol. NMR. 6:153-162.
Frey, M. N., T. F. Koetzle, F. Koetzle, M. S. Lehmann, and W. C. Hamilton. 1973. Precision neutron diffraction structure determination of protein and nucleic acid components. X. A comparison between the crystal and molecular structures of L-tyrosine and L-tyrosine hydrochloride. J. Chem. Phys. 58:2547-2556.
Frydman, L., G. C. Chingas, Y. K. Lee, P. J. Grandinetti, M. A. Eastman, G. A. Barrall, and A. Pines. 1992. Correlation of isotropic and anisotropic chemical-shifts in solids by 2-dimensional variable-angle-- spinning NMR. Isr. J. Chem. 32:161-164.
Fushman, D., R. Weisemann, H. ThUring, and H. Ruterjans. 1994. Backbone dynamics of ribonuclease-TI and its complex with 2'GMP studied by 2-dimensional heteronuclear NMR-spectroscopy. J. BiomoL NMR. 4:61-78.
Gibbs, S. J., and C. S. Johnson. 1991. A PFG NMR experiment for accurate diffusion and flow studies in the presence of eddy currents. J. Magn. Reson. 93:395-402.
Hooker, T. M. J., and J. A. Schellman. 1970. Optical activity of aromatic chromophores. I. o-, m-, and p-tyrosine. Biopolymers 9:1319-1348. lino, M., and Y. Okuda. 1997. Concentration dependence of Brownian
motion and the viscosity of hemoglobin solutions. Jpn. J. Appl. Phys. 36:3786-3790.
Ishima, R., and K. Nagayama. 1995. Protein backbone dynamics revealed by quasi spectral density function analysis of amide N-15 nuclei. Biochemistry. 34:3162-3171.
James, D. R. 1985. Fluorescence lifetime quenching and anisotropy studies of ribonuclease TI. Biochemistry. 24:5517-5526.
Jarvet, J., P. Allard, A. Ehrenberg, and A. Graslund. 1996. Spectral-density mapping of 13C"-lH" vector dynamics using Bipolar relaxation rates measured at several magnetic fields. J. Magn. Reson. B. 111:23-30.
Kalverda, A. P., M. Ubbink, G. Gilardi, S. S. Wijmenga, A. Crawford, L. J. C. Jeuken, and G. W. Canters. 1999. Backbone dynamics of azurin in solution: slow conformational change associated with deprotonation of histidine 35. Biochemistry. 38:12690-12697.
Kawato, S., K. J. Kinosita, and A. Ikegami. 1977. Dynamic structure of lipid bilayers studied by nanosecond fluorescence. Biochemistry. 16: 2319-2324.
Kay, L. E., D. A. Torchia, and A. Bax. 1989. Backbone dynamics of proteins as studied by N-15 inverse detected heteronuclear NMR-- spectroscopy: application to staphylococcal nuclease. Biochemistry. 28: 8972-8979.
Kemple, M. D., P. Buckley, P. Yuan, and F. G. Prendergast. 1997. Main chain and side chain dynamics of peptides in liquid solution from C-13 NMR: melittin as a model peptide. Biochemistry. 36:1678-1688.
Kemple, M. D., P. Yuan, K. E. Nollet, J. A. Fuchs, N. Silva, and F. G. Prendergast. 1994. 13 C NMR and fluorescence analysis of tryptophan dynamics in wild-type and two single-Trp variants of Escherichia coli thioredoxin. Biophys. J. 66:2111-2126.
Kordel, J., N. J. Skelton, M. Akke, A. G. Palmer, and W. J. Chazin. 1992. Backbone dynamics of calcium-loaded calbindin-d(9k) studied by 2-dimensional proton-detected N-15 NMR-spectroscopy. Biochemistry. 31: 4856-4866.
Kouyama, T., K. Kinosita, and A. Ikegami. 1989. Correlation between internal motion and emission kinetics of tryptophan residues in proteins. Eur. J. Biochem. 182:517-521.
Krishnan, V. V., and M. Cosman. 1998. An empirical relationship between rotational correlation time and solvent accessible surface area. J. Biomol. NMR. 12:177-182.
Kroes, S. J., G. W. Canters, G. Gilardi, A. van Hoek, and A. J. W. G. Visser. 1998. Time-resolved fluorescence study of azurin variants: conformational heterogeneity and tryptophan mobility. Biophys. J. 75: 2441-2450.
Kushwaha, P. S., and P. C. Mishra. 2000. Electronic spectra, excited-state geometries and molecular electrostatic potentials of aromatic amino acids. J. Photochem. Photobiol. 137:79-86.
Lipari, G., and A. Szabo. 1982. Model-free approach to the interpretation of nuclear magnetic resonance relaxation in macromolecules. I. Theory and range of validity. J. Am. Chem. Soc. 104:4546-4559.
Llinas, M., W. Meier, and K. Wuthrich. 1977. A carbon-13 spin lattice relaxation study of alumichrome at 25.1 MHz and 90.5 MHz. Biochim. Biophys. Acta. 492:1-11.
Loria, J. P., M. Rance, and A. G. Palmer. 1999. A relaxation-compensated Carr-Purcell-Meiboom-Gill sequence for characterizing chemical exchange by NMR. J. Am. Chem. Soc. 121:2331-2332.
MacKerell, A. D., R. Rigler, L. Nilsson, U. Hahn, and W. Saenger. 1987. Protein dynamics: a time-resolved fluorescence, energetic and molecular-dynamics study of ribonuclease-Ti. Biophys. Chem. 26:247-261.
Markley, J. L., A. Bax, Y. Arata, C. W. Hilbers, R. Kaptein, B. D. Sykes, P. E. Wright, and K. Wuthrich. 1998. Recommendations for the presentation of NMR structures of proteins and nucleic acids: IUPAC-IUBMB-- IUPAB Inter-Union Task Group on the standardization of data bases of protein and nucleic acid structures determined by NMR spectroscopy. J. Biomol. NMR. 12:1-23.
Markus, M. A., K. T. Dayie, P. Matsudaira, and G. Wagner. 1996. Local mobility within villin 14T probed via heteronuclear relaxation measurements and a reduced spectral density mapping. Biochemistry. 35: 1722-1732.
Mercier, P., L. Spyracopoulos, and B. D. Sykes. 2001. Structure, dynamics, and thermodynamics of the structural domain of troponin C in complex with the regulatory peptide 1-40 of troponin I. Biochemistry. 40: 10063-10077.
Moncrieffe, M. C., N. Juranic, M. D. Kemple, J. D. Potter, S. Macura, and F. G. Prendergast. 2000. Structure-fluorescence correlations in a single tryptophan mutant of carp parvalbumin: solution structure, backbone and side-chain dynamics. J. Mol. Biol. 297:147-163.
Nakai, T., J. Ashida, and T. Terao. 1989. Influence of small-amplitude motions on two-dimensional NMR powder patterns: anisotropic vibrations in calcium formate. Mol. Phys. 67:839-847.
Nishimoto, E., S. Yamashita, A. G. Szabo, and T. Imoto. 1998. Internal motion of lysozyme studied by time-resolved fluorescence depolarization of tryptophan residues. Biochemistry. 37:5599-5607.
Norwood, T. J. 1996. The suppression of cross-relaxation effects in H-1 relaxation measurements. J. Magn. Reson. Ser. A. 120:278-283. Norwood, T. J. 1997. Measurement of the relaxation rates of H-1 longi
tudinal modes. J. Magn. Reson. 125:265-279.
Ottiger, M., and A. Bax. 1998. Determination of relative N-HN, N-C', Ca-C' and Ca-H' effective bond lengths in a protein by NMR in a dilute liquid crystalline phase. J. Am. Chem. Soc. 120:12334-12341.
Palmer, A. G., R. A. Hochstrasser, D. P. Millar, M. Rance, and P. E. Wright. 1993. Characterization of amino acid side chain dynamics in a zinc-finger peptide using C-13 NMR spectroscopy and time-resolved fluorescence spectroscopy. J. Am. Chem. Soc. 115:6333-6345.
Palmer, A. G., N. J. Skelton, W. J. Chazin, P. E. Wright, and M. Rance. 1992. Suppression of the effects of cross-correlation between Bipolar and anisotropic chemical shift relaxation mechanisms in the measurement of spin spin relaxation rates. Mol. Phys. 75:699-711.
Peng, J. W., and G. Wagner. 1992. Mapping of spectral density-functions using heteronuclear NMR relaxation measurements. J. Magn. Reson. 98:308-332.
Peng, J. W., and G, Wagner. 1995. Frequency spectrum of NH bonds in eglin c from spectral density mapping at multiple fields. Biochemistry. 34:16733-16752.
Rigler, R., F. Claesens, and 0. Kristensen. 1985. Picosecond fluorescence spectroscopy in the analysis of structure and motion of bio-polymers. Anal. Instrum. 14:525-546.
Rigler, R., J. Roslund, and S. Forsen. 1990. Side-chain mobility in bovine calbindin-d9k: rotational motion of Tyrl3. Eur. J. Biochem. 188: 541-545.
Sahu, S. C., A. K. Bhuyan, A. Majumdar, and J. B. Udgaonkar. 2000. Backbone dynamics of barstar: a N-15 NMR relaxation study. Proteins. 41:460-474.
Seewald, M. J., K. Pichumani, C. Stowell, B. V. Tibbals, L. Regan, and M. J. Stone. 193. 2000. The role of backbone conformational heat capacity in protein stability: temperature dependent dynamics of the BI domain of streptococcal protein G. Protein Sci. 9:1177-1.
Smolyar, A., and C. F. Wong. 1999. Theoretical studies of the spectroscopic properties of tryptamine, tryptophan and tyrosine. J. Mol. Struct. (Theochem.). 488:51-67.
Swaminathan, R., N. Periasamy, J. B. Udgaonkar, and G. Krishnamoorthy. 1994. Molten globule-like conformation of barstar: a study by fluorescence dynamics. J. Phys. Chem. 98:9270-9278.
Tcherkasskaya, O., J. R. Knutson, S. A. Bowley, M. K. Frank, and A. M. Gronenborn. 2000. Nanosecond dynamics of the single tryptophan reveals multi-state equilibrium unfolding of protein GBI. Biochemistry. 39:11216-11226.
Terao, T., H. Miura, and A. Saika. 1986. bipolar SASS NMR spectroscopy: separation of heteronuclear dipolar powder patterns in rotating solids. J. Chem. Phys. 85:3816-3826.
Theret, I., S. Baladi, J. A. Cox, J. Gallay, H. Sakamoto, and C. T. Craescu. 2001. Solution structure and backbone dynamics of the defunct domain of calcium vector protein. Biochemistry. 40:13888-13$97.
along, C. Y., and M. R. Eftink. 1998. Incorporation of tryptophan analogues into staphylococcal nuclease, its V66W mutant, and delta 137-149 fragment: spectroscopic studies. Biochemistry. 37:8938-8946.
Wu, P., Y.-K. Li, P. Talalay, and L. Brand. 1994. Characterization of the three tyrosine residues of A5-3-ketosteroid isomerase by time-resolved fluorescence and circular dichroism. Biochemistry. 33:7415-7422.
Zhao, Q., C. Abeygunawardana, and A. S. Mildvan. 1996. 13 C NMR relaxation studies of backbone and side chain motion of the catalytic tyrosine residue in free and steroid-bound DS-3-ketosteroid isomerase. Biochemistry. 35:1525-1532.
Peter Damberg,* Juri Jarvet,* Peter Allard,^ Ulo Mets,^^ Rudolf Rigler,^^ and Astrid Graslund*
*Department of Biochemistry and Biophysics, Arrhenius Laboratories, Stockholm University, S-106 91 Stockholm, Sweden; ^Structural Biochemistry, Department of Biotechnology, Stockholm Center for Physics, Astronomy, and Biotechnology, The Royal Institute of Technology, S-106 91 Stockholm, Sweden; and ^^Department of Medical Biophysics, Karolinska Institute, S-171 77 Stockholm, Sweden
Address reprint requests to Dr. Astrid Graslund, Department of Biochemistry and Biophysics, Arrhenius Laboratories, Stockholm University, S-106 91 Stockholm, Sweden. Tel.: 46-8-162450; Fax: 46-8-155597; E-mail: astrid@dbb.su.se.
Copyright Biophysical Society Nov 2002
Provided by ProQuest Information and Learning Company. All rights Reserved