METHOD OF PREPARATION
1. Calculate the required quantity of each ingredient for the total amount to be prepared.
2. Accurately weigh and/or measure each ingredient.
3. Disperse the carbomer slowly by sprinkling onto about 95 mL of rapidly agitated purified water.
4. Decrease the agitation to slow mixing; add the benzalkonium chloride and mix well.
5. Add sufficient trolatnine to obtain a pH of approximately 6.
6. Add sufficient purified water to volume and mix well.
7. Package and label.
PACKAGING
Package in tight, light-resistant containers.1
LABELING
Keep out of reach of children. Use only as directed.
STABILITY
A beyond-use date of up to 6 months would be appropriate for this preparation.1
USE
Benzalkonium topical gel has been used as a topical antiseptic for the skin.
QUALITY CONTROL
Quality-control assessment can include theoretical weight compared to actual weight, pH, specific gravity, active drug assay, color, clarity, texture-surface, texture-spatula spread, appearance, feel, rheological properties and physical observations.2
DISCUSSION
Benzalkonium chloride topical gel can be used in the treatment of superficial minor skin infections and as a skin disinfectant gel, especially for individuals that may be allergic to ingredients contained in commercially available skin disinfectant products.
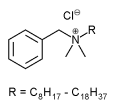
Benzalkonium chloride is a bactericidal antimicrobial agent commonly used as a preservative in many ophthalmic, otic, nasal and parenteral formulations. It occurs as a white or yellowish-white amorphous powder, a thick gel or gelatinous pieces/flakes with a characteristic mild, aromatic odor, soapy touch and very bitter taste. It is very soluble in water, alcohol and acetone. It is hygroscopic and a 10% w/v aqueous solution has a pH in the range of 5 to 8. Benzalkonium Chloride Solution NF is a clear liquid, colorless or slightly yellow unless a color has been added. It has an aromatic odor and a bitter taste. Benzalkonium chloride is composed of a mixture of straight chain homologs that possess different physical, chemical and microbiological properties. The proportions of these homologs in the mixture determine its effectiveness as a preservative and disinfectant.3
Carbopol 934P (a carbomer) is a synthetic, high-molecular-weight polymer composed of acrylic acid cross-linked with either allyl sucrose or allyl ethers of pentaerythritol. Carbomers occur as white-colored, fluffy, acidic, hygroscopic powders with a slight characteristic odor. The molecular weight of carbomer 934 is approximately 3 × 10^sup 6^. The pH of a 0.5-1.0% dispersion is in the range of 2.5 to 3.5. Carbomers are soluble in water and, after neutralization, in 95% ethanol and glycerin. When dispersed in water, an acidic colloidal solution of low viscosity will form that will thicken when an alkaline material, such as triethanolamine, is added. Effort should be made to minimize the incorporation of air bubbles into the gel. Maximum viscosity can generally be obtained in a pH range of 6 to 11.4
Trolamine (C^sub 6^H^sub 15^NO^sub 3^, MW 149.19, TEA, triethanolamine) is an alkalizing and emulsifying agent. It occurs as a variable mixture of alkanolamines and is a clear, colorless to pale yellow-colored viscous liquid with a slight ammoniacal odor. Its specific gravity is about 1.120-1.128 g/mL and it has a pH of 10.5 in a 0.1 N aqueous solution. It is very hygroscopic; it is miscible with water, 95% ethanol, methanol and acetone.5
Purified water is water that is obtained by distillation, ion exchange, reverse osmosis or some other suitable process. It is a clear, colorless, odorless and tasteless liquid. It is miscible with most polar solvents and is chemically stable in all physical states (ice, liquid and steam).6
REFERENCES
1. US Pharmacopeial Convention, Inc. United States Pharmacopeia 27-National Formulary 22. Rockville, MD: US Pharmacopeial Convention, Inc.; 2004: 2345-2349.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of ointments/creams/gels. IJPC 1998; 2: 308-309.
3. Kibbe AH. Benzalkonium chloride. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC: American Pharmaceutical Association; 2003: 45-47.
4. Koleng JJ, McGinity JW, Wilber WR. Carbomer. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC: American Pharmaceutical Association; 2003: 89-92.
5. Goskonda SR, Lee JC. Triethanolamine. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC: American Pharmaceutical Association; 2003: 663-664.
6. Ellison A, Nash RA, Wilkin MJ. Water. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC: American Pharmaceutical Association; 2003: 672-676.
Copyright International Journal of Pharmaceutical Compounding Sep/Oct 2004
Provided by ProQuest Information and Learning Company. All rights Reserved