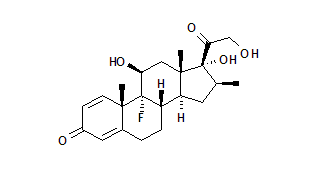
This study is currently recruiting patients. Sponsored by LEO Pharma. This study will evaluate the efficacy and safety of calcipotriol plus betamethasone dipropionate ointment compared with calcipotriol ointment in the treatment of patients with psoriasis vulgaris for a duration of 4 weeks. The study will focus on the percentage change of the psoriasis area and severity index (PASI) from baseline to the end of week 4.
Study ID Numbers: EX 0501 CN
ClinicalTrials.gov Identifier: NCT00248456
COPYRIGHT 2006 Journal of Drugs in Dermatology, Inc.
COPYRIGHT 2006 Gale Group