Asthma is a common illness in pediatric patients. Nearly all family physicians treat children with asthma. In very few other chronic conditions has management changed as much as it has for asthma in recent years. As recognition of importance of the inflammatory component in the pathogenesis of the disease has increased, emphasis on control of this component has also increased. This article reviews the 1991 recommendations of the National Heart, Lung, and Blood Institute (NHLBI)[1] in the context of the pediatric patient and includes nonpharmacologic management techniques, such as avoidance of allergens and irritants, the use of peak flow meters, and patient and family education.
Epidemiology and Pathophysiology
Mortality due to asthma has increased over the past 15 years, both in the United States and worldwide.[2] Although most deaths due to asthma occur in adults, the percentage of increase in deaths due to asthma in children is dramatic. Most authorities attribute this increased mortality to overreliance on home-administered beta agonists, resulting in inadequate or delayed therapy. The daily use (as opposed to "as needed" use) of beta agonists has been suggested as a factor in the increased mortality.[3] Black children and children who are psychologically ill have an increased risk of death from asthma.[4]
Several studies suggest that the prevalence of asthma is increasing, and this increase may partially explain the increased mortality rate. The prevalence of asthma in children and adolescents is 5 percent.[1] The number of hospitalizations for pediatric asthma has more than doubled over the past 15 to 20 years.[5] Proposed explanations for the increased prevalence of asthma include increased viral exposure in day care settings, urbanization and increased allergen exposure, particularly to dust mites.[6] Other factors are exposure to cigarette smoke,[7] pollution and other irritants. Certain respiratory viruses induce an IgE response in infants.[8]
The NHLBI defines asthma as a lung disease characterized by (1) airway obstruction or narrowing that is reversible, either spontaneously or with treatment, (2) airway inflammation and (3) airway hyperresponsiveness to various stimuli.[1] Although the etiology is undetermined, it is clear that genetics and environment are factors.
Bronchial hyperreactivity to bronchoconstrictors, such as histamine, is a feature of asthma, as is a decreased response to [beta.sub.2] sympathomimetic bronchodilators.[9] In the past, most attention to the pathophysiology and treatment of asthma was centered on bronchoconstriction. In recent years, however, focus has centered more on the inflammatory component of asthma.
Biphasic allergic response is now well recognized. The early-phase response begins when an allergen contacts antigen-specific IgE on the surface of mast cells lining the bronchi. Histamine release causes bronchoconstriction. Furthermore, histamine directly stimulates nerve fibers that heighten
bronchoconstricfion and mucus production. Vascular dilation and leakage result in local swelling. Somewhat later, release of leukotrienes, prostaglandin E, platelet activating factor and bradykinins promotes bronchoconstriction, bronchial edema, nerve fiber stimulation and attraction of inflammatory cells, including eosinophils.[9] This late-phase reaction may last several hours, and baseline hyperreactivity is heightened for several days.
Persistent inflammation causes chronic symptoms or lessens the threshold for response to triggers. The mechanisms by which nonspecific irritants trigger asthma exacerbations are less well defined than those governing IgE-mediated reactions. Irritant nerve fibers appear to release acetylcholine and neuropeptides, and to promote an influx of inflammatory cells. Edema and mucus production cause direct bronchoconstriction and propagate the vicious circle.[10,11]
Viral infections are the most common cause of exacerbations resulting in hospitalizations or emergency department visits.[12,13] Common triggers of asthma exacerbations include environmental allergens, irritants (especially cigarette smoke and pollution) and emotional upset. Other triggers are weather changes, cold air, exercise, drugs, food additives and, perhaps, sinusitis.
Pharmacologic Management of Asthma
The major pharmacologic agents used to treat asthma are bronchodilators and anti-inflammatory agents. The most commonly used bronchodilators include beta agonists and theophylline. Anticholinergics are the least commonly used bronchodilators. Anti-inflammatory agents include cromolyn (Intal), nedocromil (Tilade) and corticosteroids. Other anti-inflammatory agents are antineoplastic drugs and gamma globulin. NHLBI guidelines do not currently include the use of anticholinergics in the treatment of childhood asthma, except for limited use in acute exacerbations.
The major classes of agents used to treat asthma have not changed substantially, but their order of use has changed in recent years.
BRONCHODILATORS
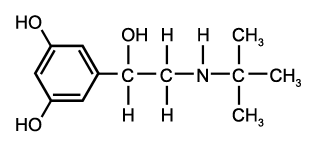
[Beta.sub.2] agonists are the most potent bronchodilator agents available. The newer agents, such as albuterol (Proventil, Ventolin), terbutaline (Brethaire, Brethine), pirbuterol (Maxaire) and salmeterol (Serevent), have greater [beta.sub.2] specificity than earlier drugs. [Beta.sub.2] agonists cause bronchodilation by direct stimulation of [beta.sub.2] receptors on smooth muscle cells. Onset of action is rapid. The agents are available in oral and inhaled forms.
In the past, beta agonists were administered on a daily basis as first-line treatment for chronic asthma. Although regular use was known to produce tolerance, this was not a major concern. In recent years, however, frequent use of [beta.sub.2] agonists has been associated with increased bronchial hyperreactivity and increased mortality.[3,11] Of particular concern is regular use of a [beta.sub.2] agonist as the only medication.
Although regular use of beta agonists has not been proved to increase the risk for fatal outcome, the current recommendation is that patients use these agents on an as needed basis rather than a routine basis.[3,11] In some children with severe asthma, use on an as needed basis may not be achievable.
Parents of children with asthma must be cautioned against overuse of beta agonists. Because this medication may provide partial relief in the face of worsening asthma, it may prevent parents from seeking early aggressive intervention. Although beta agonists generally are well tolerated, tachycardia and tremor are potential side effects.[14] The large doses usually associated with oral administration or treatment of a severe attack may cause hypokalemia.
METHYLXANTHINES
Representative methylxanthines are aminophylline, which is metabolized to the active drug theophylline, and oral theophylline. Theophylline is a slower and weaker bronchodilator than beta-adrenergic agents and lacks anti-inflammatory activity. Serum theophylline levels must be monitored periodically in patients taking this drug. Current NHLBI recommendations are to maintain theophylline levels of 5 to 15 [mu]g per mL, rather than the 10 to 20 [mu]g per mL that has traditionally been used.
Many factors affect absorption and metabolism of methylxanthines. Because of absorption problems, sustained-release formulations from reliable manufacturers are recommended. Dosages compounded by different manufacturers may not be equivalent. Theophylline metabolism varies greatly among individuals and is affected by other drugs and physiologic states[15,16] (Tables 1 and 2). Overdosage of theophylline causes nausea, vomiting, headache, and potentially fatal seizures and arrhythmias in some children.[17] Unfortunately, the seizures and arrhythmias may not be preceded by the more innocuous symptoms. Theophylline is no longer a first-line agent in the management of pediatric asthma, and it is no longer recommended for use in the emergency department.
ANTI-INFLAMMATORY AGENTS
Cromolyn. Cromolyn stabilizes the mast cell, inhibiting mast cell release and, thereby, inhibiting inflammatory cell activation.[17] It may also inhibit stimulation of irritant nerve fibers. Because cromolyn's onset of action is slow, it is useful only prophylactically. Bronchial hyperreactivity may not be decreased for six weeks after initiation of treatment. Cromolyn is more effective in mild and moderate asthma than in severe asthma and appears to be more effective in children than in adults. Currently, it is available in a metered-dose inhaler and a nebulized form. The recommended initial frequency of dosing is four times daily, although patients may tolerate subsequent reduction to twice-daily dosing.[1] Cromolyn also prevents exercise-induced asthma. It is the safest medication for day-to-day treatment of asthma.
Nedocromil. Nedocromil is a pyranoquinolone dicarboxylic acid derivative that has in vitro and in vivo anti-inflammatory and mast-cell stabilizing properties. Nedocromil has properties similar to cromolyn but is at least four to eight times more potent.[18] Nedocromil improves forced expiratory volume in one second ([FEV.sub.1]) and decreases the use of concomitant bronchodilators. It also decreases cough, and daytime and nighttime asthma symptom scores.[19]
Long-term therapy with nedocromil reduces nonspecific airway responsiveness in nonallergic pediatric asthma.[20] The usual dose is two puffs (4 mg) four times daily, which may be decreased to two puffs twice daily, with clinical response. Up to 25 percent of children may note a bad taste; otherwise, nedocromil has few side effects. It may be as safe as cromolyn, but clinical experience is too limited at this point to draw this conclusion.
Nedocromil is useful as a first-line agent in patients with moderate asthma and as an adjunct in patients with severe asthma. Use of nedocromil may allow reduction in the amount and required frequency of other medications.[20] Currently, nedocromil is approved only for children aged 12 years and older.
Corticosteroids. Corticosteroids are the most effective agents for the treatment of chronic asthma. Corticosteroids inhibit most aspects of the inflammation that occurs in patients with asthma, particularly eosinophil effects.[10,21] They enhance production of smooth muscle beta receptors and, thereby, increase the effectiveness of bronchodilator drugs.[21] Corticosteroids are available in many inhaled preparations. The most common preparations used in the United States are beclomethasone (Beclovent, Vanceril), triamcinolone (Azmacort) and flunisolide (Aerobid).
In management of patients with chronic asthma, the inhaled forms are more effective per unit dose than are the oral forms. For management of patients with acute asthma, oral or parenteral dosages must be used.[22]
Side effects of corticosteroids include susceptibility to infection, inhibition of the hypothalamic-pituitary-adrenal axis, aseptic necrosis, Cushingoid features and possible decreased bone mineralization and delayed growth. These side effects occur mainly as a result of prolonged use of systemic steroids.
Inhaled steroids have an excellent safety record in the treatment of pediatric asthma. Potential effects on growth are a concern, but a recent meta-analysis of several studies failed to show a significant effect on growth from inhaled steroids.[23] Because side effects are not of concern with cromolyn, it is preferred as the initial therapy in pediatric patients with moderate asthma. However, inhaled corticosteroids are indicated in pediatric patients with severe asthma and in patients with moderate asthma who are not responding to cromolyn. Chronic use of oral corticosteroids is reserved for patients with severe asthma that is inadequately controlled with inhaled corticosteroids and other medications. Short courses of oral corticosteroids reduce the severity of asthma exacerbations and increase the likelihood of discharge from the emergency department.[1]
Management of Chronic Asthma
The NHLBI guidelines classify asthma as mild, moderate or severe. Approximately 70 percent of asthma cases are considered mild, 25 percent moderate and 5 percent severe. Classification and management are based on chronicity of symptoms and severity of acute attacks (Table 3).
[TABULAR DATA OMITTED]
Mild Asthma. Mild asthma is characterized by symptoms occurring less than three times per week, rare nocturnal awakening and essentially normal pulmonary function tests, with an [FEV.sub.1] greater than 80 percent of the predicted value. Children with mild asthma should receive a beta agonist on an as needed basis, for wheezing. A beta agonist or cromolyn should be taken before exposure to known triggers, such as exercise or allergens.
Moderate Asthma. Moderate asthma is characterized by a need for beta agonists three or more times weekly, intermittent nocturnal awakening, an [FEV.sub.1] 60 to 80 percent of predicted value, and a 15 percent or greater response to a bronchodilator.
Children with moderate asthma should receive daily preventive therapy. Cromolyn and nedocromil are agents of first choice for these children because of infrequent toxicity, demonstrated efficacy and anti-inflammatory effects. If control is incomplete, inhaled steroids may be added or substituted, or theophylline may be added. The goal is to decrease symptoms, reduce severity and frequency of exacerbations, and allow nearnormal participation in childhood activities, such as school and sports. Beta agonists should be available for wheezing.
Severe Asthma. Severe asthma is characterized by near-daily symptoms, persistently abnormal pulmonary function tests with [FEV.sub.1] less than 60 percent of predicted value, significant exercise and activity impairment, and frequent exacerbations requiring acute care visits and hospitalizations.
Response to bronchodilator administration is incomplete. Patients usually require inhaled corticosteroids. Supplemental cromolyn, nedocromil or theophylline may be helpful. Beta agonists should be used for asthma symptoms or drops in the peak expiratory flow rate. Some children require beta agonists on a regular basis. Dosage frequencies of once or twice daily are preferable if use only as needed cannot be achieved. If inhaled steroids and the additional measures above do not result in aceptable control of asthma, oral steroids may be given. Children who are taking oral steroids on a long-term basis should be on an alternate-day schedule, if possible.
Management of Acute Asthma
Accurate assessment of asthma severity helps guide therapy. The following parameters help in the assessment of the severity of asthma exacerbation in children: peak expiratory flow rate (PEFR), respiratory rate, alertness, dyspnea, accessory muscle use, color, wheezing, oxygen saturation and partial pressure of carbon dioxide ([Pco.sub.2]) (Tables 4 and 5). The child's response to treatment must be continually reassessed. Systemic steroids are recommended if response to beta agonists is incomplete. The NHLBI protocol for management of acute asthma is presented in Figure 1. Table 6 lists dosages for acute exacerbations. Written instructions for the treatment plan and follow-up plan are helpful to young patients.
[TABULAR DATA OMITTED]
Nonpharmacologic Management
ALLERGEN AND IRRITANT AVOIDANCE
The avoidance of known inducers of asthma exacerbations, such as cigarette smoke and allergens, is important (see patient information handout). Children who fail to respond to allergen avoidance measures and medications may be helped by specific allergen immunotherapy (allergy "shots"). Several recent studies[24] have documented success with immunotherapy for patients with allergic asthma. The decision to begin immunotherapy should be based on the child's degree of control, medication requirements and side effects, and the success of avoidance measures.
PEAK FLOW METERS
Peak flow meters are extremely useful for monitoring a child's asthma, both in the office and at home. Asymptomatic changes in peak flow often precede deterioration, and early treatment can prevent problems from becoming serious. When baseline peak flow values are not known, the child's current peak flow can be compared with normative data. However, because the normal range is wide, it is more useful to compare the current peak flow to the child's highest previous peak flow. Instructions for use of the peak flow meter are given in the accompanying patient information handout. Peak flow meters greatly enhance the physician's ability to give a clinical assessment by telephone of an asthma exacerbation and the patient's response to treatment (Figure 2).
FAMILY AND PATIENT EDUCATION
Although many asthmatic children have clinical remission later in childhood or in adolescence, bronchial hyperreactivity may persist. While it is not possible to predict which children will have persistent disease, the child with severe asthma will probably have lifelong problems. It is therefore important to help the asthmatic child build a good foundation of healthful habits and appropriate management that will last throughout adulthood. The physician must take an interactive role, addressing the many factors affecting quality of life for the asthmatic child (Table 7).[25]
[TABULAR DATA OMITTED]
A developmentally oriented model for primary care of children with asthma includes three levels of responsibility: (1) medical intervention and management, (2) provision for developmental, psychosocial and emotional needs, and (3) integration of the treatment plan into family, school and social life.[26] Many educational programs are available to physicians, patients and patients' families[27] (Table 8). Such programs enable children and their families to obtain accurate information, overcome fear, decrease disruption of family life and increase confidence in managing asthma on a day-to-day basis.
REFERENCES
[1.] National Asthma Education Program. Expert Panel. Guidelines for the diagnosis and management of asthma. Bethesda, Md.: National Heart, Lung, and Blood Institute, 1991; DHHS publication no. 91-3042. [2.] Asthma - United States, 1980-1987. MMWR Morb Mortal Wkly Rep 1990;39:493-7. [3.] Spitzer WO, Suissa S, Ernst P, Horwitz RI, Habbick B, Cockcroft D, et al. The use of beta-agonists and the risk of death and near death from asthma. N Engl J Med 1992;326:501-6. [4.] Weiss KB, Wagener DK. Changing patterns of asthma mortality. Identifying target populations at high risk. JAMA 1990;264:1683-7. [5.] Evans R 3d, Mullally DI, Wilson RW, Gergen PJ, Rosenberg HM, Grauman JS, et al. National trends in the morbidity and mortality of asthma in the US. Prevalence, hospitalization and death from asthma over two decades: 1965-1984. Chest 1987;91(Suppl 6):65-74S. [6.] Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med 1990;323:502-7. [7.] Martinez FD, Cline M, Burrows B. Increased incidence of asthma in children of smoking mothers. Pediatrics 1992;89:21-6. [8.] Busse WW, Lemanske RF Jr, Dick EC. The relationship of viral respiratory infections and asthma. Chest 1992;101 (Suppl 6):385-8. [9.] Sheth KK, Lemanske RF Jr. Pathogenesis of asthma. Pediatrician 1991;18:257-68. [10.] Barnes PJ. A new approach to the treatment of asthma. N Engl J Med 1989;321:1517-27. [11.] Sears MR, Taylor DR, Print CG, Lake DC, Li QQ, Flannery EM, et al. Regular inhaled beta-agonist treatment in bronchial asthma. Lancet 1990;336: 1391-6. [12.] McIntosh K, Ellis EF, Hoffman LS, Lybass TG, Eller JJ, Fulginiti VA. The association of viral and bacterial respiratory infections with exacerbations of wheezing in young asthmatic children. J Pediatr 1973;82:578-90. [13.] Horn ME, Gregg I. Role of viral infection and host factors in acute episodes of asthma and chronic bronchitis. Chest 1973;63(Suppl):44-8S. [14.] Crane J, Burgess C, Beasley R. Cardiovascular and hypokalaemic effects of inhaled salbutamol, fenoterol, and isoprenaline. Thorax 1989;44:136-40. [15.] Jonkman JH, Upton RA. Pharmacokinetic drug interactions with theophylline. Clin Pharmacokinet 1984;9:309-34. [16.] Paloucek FP, Rodvold KA. Evaluation of theophylline overdoses and toxicities. Ann Emerg Med 1988;17:135-44. [17.] Foreman JC, Garland LG. Cromoglycate and other antiallergic drugs: a possible mechanism of action. Br Med J 1976;1:820-1. [18.] Richards R, Phillips GD, Holgate ST. Nedocromil sodium is more potent than sodium cromoglycate against AMP-induced bronchoconstriction in atopic asthmatic subjects. Clin Exp Allergy 1989; 19:285-91. [19.] North American Tilade Study Group. A double-blind multicenter group comparative study of the efficacy and safety of nedocromil sodium in the management of asthma. Chest 1990;97:1299-306. [20.] Bel EH, Timmers MC, Hermans J, Dijkman JH, Sterk PJ. The long-term effects of nedocromil sodium and beclomethasone dipropionate on bronchial responsiveness to methacholine in nonatopic asthmatic subjects. Am Rev Respir Dis 1990;141: 21-8. [21.] Kaliner M. Mechanisms of glucocorticosteroid action in bronchial asthma. J Allergy Clin Immunol 1985;76(2 Pt 2):321-9. [22.] Godfrey S, Konig P. Treatment of childhood asthma for 13 months and longer with beclomethasone dipropionate aerosol. Arch Dis Child 1974;49:591-6. [23.] Allen DB, Mullen M, Mullen B. A meta-analysis of the effect of oral and inhaled corticosteroids on growth. J Allergy Clin Immunol 1994;93:967-76. [24.] Bousquet J, Michel F. Specific immunotherapy in asthma: is it effective? J Allergy Clin Immunol 1994;94:1-11. [25.] Christie MJ, French D, Weatherstone L, West A. The patients' perceptions of chronic disease and its management: psychosomatics, holism and quality of life in contemporary management of childhood asthma. Applied Psychology Research Group. Psychother Psychosom 1991;56:197-203. [26.] Miller BD, Wood BL. Childhood asthma in interaction with family, school, and peer systems: a developmental model for primary care. J Asthma 1991;28:405-14. [27.] Evans D, Mellins RB. Educational programs for children with asthma. Pediatrician 1991;18:317-23.
JOHN E. MOFFITT, M.D. is associate professor in the Department of Pediatrics, Division of Allergy an Immunology, at the University of Mississippi School of Medicine, Jackson. Dr. Moffitt graduated from the University of Mississippi School of Medicine and completed a pediatric residency there and a fellowship in allergy and immunology at the Medical College of Georgia School of Medicine in Augusta.
JUDITH G. GEARHART, M.D. is associate professor and director of the student division in the Department of Family Medicine at the University of Mississippi School of Medicine. After graduating from the University of Mississippi School of Medicine, she completed a family medicine residency there.
ANNE B. YATES, M.D. is assistant professor in the Department of Pediatrics, Division of Allergy and Immunology, at the University of Mississippi School of Medicine. She graduated from the University of Mississippi School of Medicine, where she completed a pediatric residency and was chief resident in pediatrics. She also completed a fellowship in allergy and immunology at Baylor College of Medicine in Houston.
COPYRIGHT 1994 American Academy of Family Physicians
COPYRIGHT 2004 Gale Group