Study objective: Adding inhaled long-acting [[beta].sub.2]-agonists to a low dose of inhaled corticosteroids (ICSs) results in better asthma control than increasing the dose of ICSs. An important, but as yet unresolved, question is whether this is due to an additional reduction of airway inflammation.
Design: Double-blind, parallel-group trial.
Patients: Forty asthma patients (FE[V.sub.1], 50 to 90% predicted; provocative concentration of a substance [methacholine] causing a 20% fall in FE[V.sub.1] of < 8 mg/mL; no ICSs in the last 4 weeks).
Interventions: Randomization to 8 weeks of treatment with 100 [micro]g of budesonide bid plus placebo (BUD200) or 100 [micro]g of budesonide bid plus 12 [micro]g of formoterol (BUD200 + F). Then the dose of budesonide (BUD) was increased to 400 [micro]g bid in both groups for another 8 weeks. Bronchial biopsy specimens were collected before, and after 8 and 16 weeks of treatment. Eosinophils (major basic protein [MBP]) and mast cells (tryptase) were analyzed by immunohistochemistry.
Results: BUD200 reduced the MBP staining (p = 0.008) and tryptase staining (p = 0.048) in the epithelium compared to baseline levels. There were no significant differences between the BUD200 and BUD200 + F groups. In both groups, increasing the dosage of BUD to 800 [micro]g had no significant additional antiinflammatory effect.
Conclusions: Our results demonstrate that BUD administered at a low dose has significant antiinflammatory effects in patients with mild asthma. No significant additional antiinflammatory effects could be demonstrated either by adding formoterol or by increasing the dose of BUD.
Key words: airway inflammation; asthma; bronchial biopsy; budesonide; formoterol
Abbreviations: BHR = bronchial hyperreactivity; BUD = budesonide; BUD200 = 100 [micro]g of budesonide bid plus placebo; BUD200 + F = 100 [micro]g of budesonide bid plus 12 [micro]g of formoterol bid; BUD800 = 400 [micro]g of budesonidebid; BUD800 + F = 400 [micro]g of budesonide bid plus 12 [micro]g of formoterol bid; CI = confidence interval; eNO = exhaled nitric oxide; ICS = inhaled corticosteroid; LA = long-acting; MBP = major basic protein; MCh = methacholine; P[C.sub.20] = provocative concentration of a substance causing a 20% fall in FE[V.sub.1]
**********
Asthma is a chronic disease that is characterized by reversible airflow obstruction and bronchial hyperreactivity (BHR) to a variety of stimuli. Considerable evidence has been obtained to show that airway inflammation is a major factor in the pathogenesis of asthma and associated BHR. The inflammatory pattern in asthma is multicellular in nature, with mainly mast cells, eosinophils, and T lymphocytes participating in the response. An important therapeutic objective in treating asthma patients is to control the underlying inflammatory process in the airways. Current guidelines advocate the use of inhaled corticosteroids (ICSs). (1) Treatment with ICSs not only leads to consistent reductions in the number and activation of mast cells and eosinophils in biopsy specimens, (2,3) it also reduces BHR.
ICSs are generally combined with inhaled short-acting and/or long-acting (LA) [[beta].sub.2]-agonists for symptomatic relief in patients with asthma. LA [[beta].sub.2]-agonists are considered to be smooth muscle relaxants, while their antiinflammatory properties are still a matter of debate. Although the antiinflammatory effects of [[beta].sub.2]-agonists have been demonstrated clearly in in vitro experiments, these have not been proven in vivo. Nevertheless, several studies (4-6) have indicated that adding inhaled LA [[beta].sub.2]-agonists to a low close of ICSs results in better asthma control than increasing the dose of ICSs with respect to asthma symptoms, lung function, and the number of exacerbations. An important, but as yet unresolved, question is whether this is due to an additional reduction of airway inflammation. We set up a double-blind, parallel-group trial to answer this question. The effect of 8 weeks of treatment with 100 [micro]g of budesonide bid plus placebo (BUD200) compared with 100 [mciro]g of budesonide bid plus 12 of [micro]g formoterol bid (BUD200 + F), followed by another 8 weeks of treatment with 400 [micro]g bid with or without formoterol on markers of inflammation in bronchial biopsy specimens and in exhaled air was studied.
MATERIALS AND METHODS
Subjects
Forty nonsmoking both atopic and nonatopic asthma patients, who were receiving a maximum dose of 800 [micro]g of ICSs daily prior to the run-in, were selected if they met the following criteria: 18 to 55 years of age, FE[V.sub.1] between 50% and 90% of predicted normal; provocative concentration of a substance (methacholine [MCh]) causing a 20% fall in FE[V.sub.1] (P[C.sub.20]) of < 8 mg/mL, as measured by a 2-min tidal breathing method7 Atopy was defined as having a positive radioallergosorbant test for house dust mite or at least two other common inhaled allergens. All subjects were in stable condition, were nonsmokers, and were treated according to international guidelines. (8) All participants gave informed written consent to the study, which was approved by the medical ethics committee of the Erasmus Medical Center, Rotterdam, the Netherlands. The study was conducted according to the principles stated in the Declaration of Helsinki.
Study Design
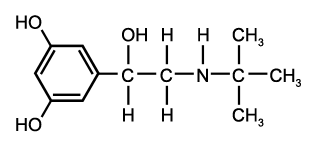
The study was a randomized, double-blind, parallel-group design. After a run-in period of 4 weeks, subjects entered an 8-week treatment period receiving 100 [micro]g of budesonide (Pulmicort Turbuhaler; AstraZeneca; Sodertalje, Sweden) bid plus placebo (ie, BUD200) or 100 [micro]g of budesonide bid plus 12 [micro]g of formoterol (Oxis Turbuhaler; AstraZeneca) bid (ie, BUD200 + F). Then, the dosage of budesonide (BUD) was increased to 400 p,g bid in all patients for another 8 weeks (BUD800 group), while the dosage of formoterol, 12 [micro]g bid, was continued in the randomized group (BUD800 + F).
At the start of the 4-week run-in period, all medication was stopped. Patients were allowed to receive only the inhaled short-acting [[beta].sub.2]-agonist terbutaline (Bricanyl Turbuhaler; AstraZeneca), 0.5 mg per dose, on an as-needed basis. Treatment with terbutaline was allowed up to 8 h prior to making the study measurements.
At the start of the study period, at visit 1, a radioallergosorbant test and spirometry were performed. Reversibility was tested by measuring FE[V.sub.1] relative to baseline following the inhalation of four puffs of 0.25 mg of terbutaline using a metered-dose inhaler (Nebuhaler; AstraZeneca).
At the second visit to the clinic 3 to 7 days later, exhaled nitric oxide (eNO) levels and P[C.sub.20] for MCh were determined. All patients fulfilling the inclusion criteria were scheduled for bronchoscopy at least 1 week after the baseline visit. Following bronchoscopy, patients were randomized to one of the treatment regimens.
After 7 weeks of treatment, spirometry, eNO level measurement, and the determination of the P[C.sub.20] for MCh were repeated. One week later, after exactly 8 weeks, a second bronchoscopy was performed. Then, the dosage of BUD was increased to 400 [micro]g bid in all patients for another 8 weeks. After 7 weeks of the second treatment period, spirometry, eNO level measurement, and the determination of the P[C.sub.20] for MCh were repeated. After exactly 8 weeks, a third bronchoscopy was performed. Eight hours prior to the pulmonary function tests, only the 0.5-mg (as needed) dose of terbutaline (Turbuhaler; AstraZeneca) was discontinued.
Bronchoprovocation
MCh was administered according to a standardized 2-min tidal-breathing method.7 The aerosols were generated using a nebulizer (model 646; DeVilbiss Health Care; Somerset, PA) [output, 0.13 mL/min].
eNO
The amount of eNO was measured by means of chemiluminescence (Aerocrine NO System; Aeroerine AB; Danderyd, Sweden).9 After complete exhalation, subjects inhaled nitric oxide-free air, via a filter (Purafil; Doraville, GA), to total lung capacity and were subsequently asked to exhale slowly at a constant flow rate between 0.075 and 0.125 L/s for 10 s. Measurements were performed at least in triplicate in order to reach an accuracy of 5 to 10% for nitric oxide levels.
Bronchial Biopsies
All bronchial biopsies were performed by the same pulmonary physician (S.E.O.). The fiberoptic bronchoscope (BF, type P20 D; Olympus; Tokyo, Japan) was introduced orally under local anesthesia. Mucosal biopsy specimens (three to six) were obtained from the segmental carinae and were embedded in a cutting compound (Tissue-Tek II Optimal Cutting Temperature compound; Sakura Finetek USA Inc; Torrance, CA), which was frozen and stored at -80[degrees]C.
Immunohistochemistry
Once all biopsy specimens were collected, 6-[micro]m serial tissue sections were cut on a cryostat (model HM-560; Microm; Heidelberg, Germany). At least two sections 120 [micro]m apart from one biopsy specimen were placed on a poly-L-lysine-coated microscopic slide (Sigma Diagnostics; St. Louis, MO). Next, sections were air-dried for 30 min and stored at -80[degrees]C until use. Immunostaining was carried out with the following substances: [alpha]-major basic protein (MBP [clone BMK13]) [Sanbio BV; Uden, the Netherlands]; and [alpha]-tryptase (Chemicon Brunschwig Chemie; Temecula, CA). (10) The binding of the antibodies was detected by the immunoalkaline phosphatase antialkaline phosphatase method.
Positively stained areas in the lamina propria (depth, 100 [micro]m below the reticular basement membrane) were quantified using an image analysis system (Quantimed; Leica; Rijswijk, the Netherlands). Images were taken at 10 x 10 magnification. A minimum area of 100,000 [micro][m.sup.2] was analyzed per immunostaining for a given subject at a given time point. Thresholds for hue; saturation, and intensity of color were set for each immunohistochemical stain, and these were kept constant during the analysis of the entire sample set. This approach "allows for a densitometric analysis. The ratio of the positive stained area divided by the total area analyzed was taken as a measure for each immunohistochemical staining. This procedure has been recorded in a standard operating procedure and was performed by at least two independent observers to minimize bias. This method has been validated by Sont et al. (11)
Statistical Analysis
For differences in efficacy between the two treatment groups, P[C.sub.20] ([log.sub.2]-transformed), FE[V.sub.1], and eNO (in-transformed) were parametrically analyzed using analysis of covariance, with the baseline measurement as the covariate. Mean changes within either treatment group were tested using paired t tests.
For the inflammatory cells and markers, changes from baseline after logarithmic transformation were compared between the two treatment groups using the Mann-Whitney test. Changes within either treatment group were tested using paired Wilcoxon tests after logarithmic transformation. Nonparametric 95% confidence intervals (CIs) were estimated according to the method of Hodges and Lehmann (12) for the efficacy ratio between the two treatments. The MBP-positive cells were predetermined to be the primary outcome variable. A significance level of 0.05 was used.
RESULTS
All subjects who entered the study, completed it (21 men, 19 women; mean age, 28.8 years; age range, 19 to 52 years). Seventeen of 20 patients in each group were atopic. The mean reversibility rate was 16.4%. There were no significant differences for the baseline values between the groups. No adverse events were reported. Prior to the run-in period, patients used a mean dose of 520 [micro]g of ICSs daily; only one patient had not used ICSs prior to inclusion in the study.
Spirometry
In the BUD200 group as well as in the BUD200 + F group, FE[V.sub.1] (percent predicted) increased significantly after 8 weeks compared to baseline (Table 1). The baseline-adjusted mean difference of 3.1% points in change between the BUD200 + F and BUD200 groups was not significant (95% CI, -2.3 to 8.6; p = 0.26) [Table 2].
Increasing BUD200 to BUD800 led to a further significant increase in FE[V.sub.1], whereas increasing BUD200 + F to BUD800 + F did not produce a further significant change in FE[V.sub.1]. The baseline-adjusted mean difference of -3.1% shows that the difference between the treatments was not significant (95% CI, -7.8 to 1.6; p = 0.19).
Bronchoprovocation
In both the BUD200 group and the BUD200 + F group, the change in the P[C.sub.20] for MCh after 8 weeks was highly significant. The baseline-adjusted mean difference in the change between the BUD200 + F and BUD200 groups was not significant (p = 0.14). Increasing the daily dose of BUD from 200 to 800 [micro]g did not result in a further change of the mean P[C.sub.20] for MCh in any of the treatment groups. The mean difference in a change of -0.7 doubling concentration between the treatments was not significant (95% CI, -1.7 to 0.3; p = 0.15).
eNO
eNO decreased significantly in both the BUD200 and the BUD200 + F groups after 8 weeks of treatment. There was no significant difference that was detectable between the two groups (p = 0.58). Increasing the dose of steroids to 800 [micro]g resulted in a significant decrease in eNO in the BUD200 group, while there was no such effect detectable in the BUD200 + F group. There was no significant difference between the two treatment groups after increasing the dose of BUD (p = 0.74) [Fig 1].
[FIGURE 1 OMITTED]
Bronchial Biopsies
In the epithelium, MBP was significantly reduced in the BUD200 group after 8 weeks of treatment, while this was not the ease in the BUD200 + F group (Table 1). The difference in the median change from baseline between the BUD200 and BUD200 + F groups was not significant (p = 0.28).
Increasing the close of steroids to 800 [micro]g did not significantly change MBP in either of the treatment groups. No significant difference between treatments could be detected after increasing the BUD dose to 800 [micro]g (p = 0.86) [Fig 2, top, A].
[FIGURE 2 OMITTED]
In the subepithelium, the median MBP decreased significantly in both file BUD200 and the BUD200 + F groups after 8 weeks. The difference in the median change from baseline between the BUD200 and BUD200 + F groups was not significant (p = 0.34). Increasing BUD200 to BUD800 in both groups did not significantly change MBP density within groups or between groups (p = 0.97) [Fig 2, bottom, B).
Concerning mast cells, BUD200 resulted in a significant reduction of tryptase in the epithelium compared to baseline, but it hardly affected tryptase staining in the subepithelium. BUD200 + F resulted in nonsignificant reductions of tryptase staining in both the epithelium and subepithelium compared to baseline (Fig 3, top, A). No significant differences between treatments were detectable for any of the variables, or after increasing the BUD dose to 800 [micro]g (Fig 3, bottom, B).
[FIGURE 3 OMITTED]
DISCUSSION
Several authors (5,13) have demonstrated the clinical benefit of adding LA [[beta].sub.2]-agonists as maintenance treatment to inhaled ICSs in asthmatic patients. The mechanism by which the addition of LA [[beta].sub.2]-agonists leads to better control in asthma has not been fully elucidated yet but may be due to an enhanced antiinflammatory effect.
Both in vitro and in vivo animal data have suggested that LA [[beta].sub.2]-agonists have some antiinflammatory effect. An inhibitory effect on the infiltration of inflammatory cells in the skin and lungs of guinea pigs has been demonstrated. (14,15) It has also been shown that the exudation of plasma from postcapillary venules, which is an important component of acute inflammation, is inhibited by LA [[beta].sub.2]-agonists. (14) Others have shown (16,17) additive and synergistic effects on the inflammatory properties of resident pulmonary cells, whereas some authors (18) have reported interactions between [[beta].sub.2]-agonists and glucocorticoids at the mechanistic level. A synergistic effect of BUD and formoterol was suggested. (4) Our results do not support this suggestion, probably because treatment with low-dose BUD alone already resulted in a significant decrease of inflammatory cells in bronchial biopsy specimens accompanied by a significant improvement in lung function. This is underlined by the fact that we could not demonstrate an additional antiinflammatory effect even after quadrupling the ICS dose. We conclude from these findings that our patients with mild asthma who were receiving the low dose of BUD were close to the top of their dose-response curve for ICSs, allowing no room for significant improvement. Also Jatakanon et al (19) have reported that treatment with low doses of steroids leads to a significant reduction in the levels of markers of inflammation.
Previously, other investigators (13) have demonstrated similar effects of low-dose ICSs in steroid-naive patients with mild asthma. However, they were able to show (13) an additive clinical effect of formoterol in the group of asthma patients receiving low doses of ICSs. In line with this finding, it was demonstrated that the addition of salmeterol resulted in a greater improvement in lung function and symptom control than did doubling the dose of beclomethasone dipropionate. (5) Our patients were atopic asthma patients using up to 800 [micro]g of ICSs daily prior to the run-in period during which they were taken off steroid therapy. While not receiving steroids, it is fair to assume that the condition of the airways deteriorated and the inflammation increased at the same time. At the end of the run-in period, the severity of the airway inflammation had reached a level at which a low dose of BUD was sufficient to reduce the airway inflammation to such a degree that no further reduction could be expected.
The Formoterol and Corticosteroids Establishing Therapy study (4,20) looked at the number of exacerbations that occurred during a 12-month study in which formoterol was added to both low-dose and high-dose BUD in patients with asthma. The addition of formoterol decreased the incidence of mild and severe exacerbations in both groups. These studies demonstrated the clinical effects of the addition of an LA [[beta].sub.2]-agonist to ICSs. Their findings are in line with those of Zetterstrom et al (21) who showed that during the first 30 days of treatment therapy with BUD and formoterol in a single inhaler tended to show more rapid improvement in lung function and symptom score compared to therapy with the same drugs in separate inhalers. It is unclear, however, whether these improvements in lung function were accompanied by a reduction of inflammation.
Only a few authors have investigated the antiinflammatory effect of LA [[beta].sub.2]-agonists in humans. In patients with mild asthma, 8 weeks of monotherapy with formoterol significantly reduced the number of submucosal mast cells. (22) It has been reported (23) that 2 weeks of treatment with formoterol significantly reduced the number of eosinophils (EG[2.sup.+]) in the submucosa and epithelium in patients with mild atopic asthma. Other investigators (6) could not confirm such an antiinflammatory effect as measured in induced sputum when they compared 1 year of treatment with formoterol and 200 [micro]g of BUD with 800 [micro]g of BUD. Clinical asthma control was not significantly different between the two groups.
eNO as a noninvasive marker of airway inflammation has been shown (24,25) to be useful in monitoring disease activity and the effectiveness of antiinflammatory treatment. Although eNO decreased significantly after treatment with both BUD200 and BUD200 + F, we found no differences between the two treatments or after increasing the BUD dose to 800 [micro]g/d, which was in line with the decrease in inflammatory cells in the bronchial mucosa.
In contrast to some other studies, only a limited number of patients could be included in this study due its invasive nature, requiring multiple biopsies within a relatively short period of time. Therefore, a parallel-group design was considered to be a statistically valid approach. Since our main objective was to look at the change in inflammatory marker levels, neither symptom scores nor rescue [[beta].sub.2]-agonist medication were recorded by the patients.
In summary, treatment with BUD at a low dose already has significant antiinflammatory effects in the bronchial mucosa of patients with mild asthma. No significant additional antiinflammatory effects (on the inflammatory markers analyzed in this study) could be demonstrated either by adding formoterol or by increasing the dose of BUD.
ACKNOWLEDGMENT: The authors thank the Lung Function Laboratory and coworkers of the Pulmonary Research Department of the Erasmus Medical Center Rotterdam for their valuable participation in this study. The authors thank Dr. Huib Kerstjens for reading the manuscript, and for his constructive suggestions and comments.
REFERENCES
(1) Boulet LP. Comment of "What is new since the last (1999) Canadian Asthma Consensus Guidelines [letter]?" Can Respir J 2001; 8:382
(2) Burke CM, Sreenan S, Pathmakanthan S, et al. Relative effects of inhaled corticosteroids on immunopathology and physiology in asthma: a controlled study. Thorax 1996; 51: 993-999
(3) Barnes NC, Burke CM, Poulter LW, et al. The anti-inflammatory profile of inhaled corticosteroids: biopsy studies in asthmatic patients. Respir Med 2000; 94(suppl):S16-S21
(4) Pauwels RA, Lofdahl CG, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma: Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med 1997; 337:1405-1411
(5) Woolcock A, Lundback B, Ringdal N, et al. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. Am J Respir Crit Care Med 1996; 153:1481-1488
(6) Kips JC, O'Connor BJ, Inman MD, et al. A long-term study of the antiinflammatory effect of low-dose budesonide plus formoterol versus high-dose budesonide in asthma. Am J Respir Crit Care Med 2000; 161:996-1001
(7) Hargreave FE, Sterk P, Adelroth EC, et al. Airway responsiveness to histamine or methacholine: advances in measurement and interpretation. Respiration 1986; 50:72-76
(8) Busse WW, Lenfant C, Lemanske RF Jr. Asthma guidelines: a changing paradigm to improve asthma care. J Allergy Clin Immunol 2002; 110:703-705
(9) Kharitonov S, Alving K, Barnes PJ. Exhaled and nasal nitric oxide measurements: recommendations; The European Respiratory Society Task Force. Eur Respir J 1997; 10:1683-1693
(10) van den Toorn LM, Overbeek SE, de Jongste JC, et al. Airway inflammation is present during clinical remission of atopic asthma. Am J Respir Crit Care Med 2001; 164:2107-2113
(11) Sont JK, De Boer WI, van Schadewijk WA, et al. Fully automated assessment of inflammatory cell counts and cytokine expression in bronchial tissue. Am J Respir Crit Care Med 2003; 167:1496-1503
(12) Hodges JL, Lehmann EL. Estimates of location based on rank tests. Ann Math Stat 1963; 34:598-611
(13) O'Byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001; 164:1392-1397
(14) Whelan CJ, Johnson M, Vardey CJ. Comparison of the anti-inflammatory properties of formoterol, salbutamol and salmeterol in guinea-pig skin and lung. Br J Pharmacol 1993; 110:613-618
(15) Bolton PB, Lefevre P, McDonald DM. Salmeterol reduces early- and late-phase plasma leakage and leukocyte adhesion in rat airways. Am J Respir Crit Care Med 1997; 155:1428-1435
(16) Pang L, Knox AJ. Synergistic inhibition by beta(2)-agonists and corticosteroids on tumor necrosis factor-alpha-induced interleukin-8 release from cultured human airway smoothmuscle cells. Am J Respir Cell Mol Biol 2000; 23:79-85
(17) Pang L, Knox AJ. Regulation of TNF-alpha-induced eotaxin release from cultured human airway smooth muscle cells by [[beta].sub.2]-agonists and corticosteroids. FASEB J 2001; 15:261-269
(18) Adcock IM, Stevens DA, Barnes PJ. Interactions of glucocorticoids and [[beta].sub.2]-agonists. Eur Respir J 1996; 9:160-168
(19) Jatakanon A, Kharitonov S, Lim S, et al. Effect of differing doses of inhaled budesonide on markers of airway inflammation in patients with mild asthma. Thorax 1999; 54:108-114
(20) Tattersfield AE, Postma DS, Barnes PJ, et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations; The FACET International Study Group. Am J Respir Crit Care Med 1999; 160:594-599
(21) Zetterstrom O, Buhl R, Mellem H, et al. Improved asthma control with budesonide/formoterol in a single inhaler, compared with budesonide alone. Eur Respir J 2001; 18:262-268
(22) Wallin A, Sandstrom T, Soderberg M, et al. The effects of regular inhaled formoterol, budesonide, and placebo on mucosal inflammation and clinical indices in mild asthma. Am J Respir Crit Care Med 1999; 159:79-86
(23) Wallin A, Sandstrom T, Cioppa GD, et al. The effects of regular inhaled formoterol and budesonide on preformed Th-2 cytokines in mild asthmatics. Respir Med 2002; 96:1021-1025
(24) Kharitonov SA, Donnelly LE, Montusehi P, et al. Dose-dependent onset and cessation of action of inhaled budesonide on exhaled nitric oxide and symptoms in mild asthma. Thorax 2002; 57:889-896
(25) van Rensen EL, Straathof KC, Veselic-Charvat MA, et al. Effect of inhaled steroids on airway hyperresponsiveness, sputum eosinophils, and exhaled nitric oxide levels in patients with asthma. Thorax 1999; 54:403-408
* From the Departments of Pulmonary Medicine (Drs. Overbeek, Hoogsteden, and Prins, and Ms. Baelemans) and Epidemiology & Biostatistics (Dr. Mulder), Erasmus Medical Center, Rotterdam, the Netherlands.
All authors have disclosed all pertinent involvement in AstraZeneca, which has a direct financial interest in the subject of the manuscript. This study was supported by AstraZeneca, the Netherlands (study BN-00P-0059).
Manuscript received August 5, 2004; revision accepted February 14, 2005.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal. org/misc/reprints.shtml).
Correspondence to: Shelley E. Overbeek, MD, PhD, Department of Pulmonary Medicine, SV020, Erasmus Medical Center, Dr. Molewaterple-in 40, 3015 GD Rotterdam, the Netherlands; e-mail: s.e. overbeek@erasmusmc.nl
COPYRIGHT 2005 American College of Chest Physicians
COPYRIGHT 2005 Gale Group