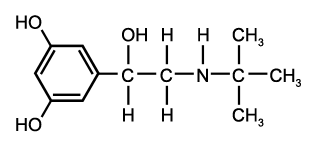
Objective: The study was undertaken to investigate the influence of once-daily treatment with montelukast (Singulair; MSD; Glattbrugg, Switzerland) on levels of exhaled nitric oxide (eNO) and lung function in preschool children with asthma.
Methods: A total of 30 children (19 girls), 2 to 5 years of age, in whom asthma had been newly diagnosed, who had a positive first-degree family history of asthma and a positive allergy test result, were allocated to undergo a 1-week run-in period of montelukast treatment, eNO and airway resistance were measured in all patients before (visit 1) and after the run-in period (visit 2), and after treatment with montelukast (4 mg once daily) for 4 weeks (visit 3).
Results: There were no significant differences in all parameters before and after the run-in period. However, the mean (SD) levels of eNO and the mean (SD) levels of airway resistance after treatment at visit 3 were 11.6 parts per billion (ppb) [9.5 ppb] and 1.15 kPa/L/s (0.26 kPa/L/s), respectively, and were significantly lower compared to values of 33.1 ppb (12.0 ppb) and 1.28 kPa/L/s (0.23 kPa/L/s), respectively, before treatment (p < 0.001) and at visit 2 (p = 0.01). There was no significant change in mean bronchodilator responsiveness between visit 3 (13.2%; SD, 6.8%) and visit 1/visit 2 (13.3%; SD, 7.0%; p = 0.47).
Conclusion: We have shown that montelukast has a positive effect on lung function and airway inflammation as measured by nitric oxide level in preschool children with allergic asthma.
Key words: airway resistance; asthma; bronchodilator responsiveness; inflammation; lung function test; montelukast; nitric oxide; preschool children
Abbreviations: eNO = exhaled nitric oxide; LTRA = leukotriene receptor antagonist; NO = nitric oxide; ppb = parts per billion; RAST = radioallergosorbent test; Rint = airway resistance measured by interrupter technique
*********
Studies investigating the effect of antiinflammatory treatment in young children with asthma have shown inconsistent results. Prophylactic inhaled antiinflammatory therapy is not effective in preventing acute exacerbations in young children with viral-induced recurrent wheeze. (1) In contrast, in children with recurrent wheeze therapy with systemic and inhaled steroids have been shown to be effective in the treatment of acute symptoms. (2,3) The different response to antiinflammatory treatment may be explained by the heterogeneity of disease expression. Most young children with recurrent wheeze experience recurrent episodes of viral-induced wheeze, and only a small number of young children experiencing recurrent or chronic wheeze have allergic asthma. (4) It may well be that the allergic asthma subgroup of children with recurrent wheeze are more likely to respond to antiinflammatory treatment. Therefore, it would be important to distinguish between the two groups of young children with recurrent wheeze. (5) However, the diagnosis of early childhood respiratory symptoms is often based on clinical history, physical examination findings, and nonspecific laboratory test results. A family history of asthma and a positive allergy test result may be helpful in making the diagnosis. (4) Over the past years, new diagnostic procedures have been developed. The measurement of exhaled nitric oxide (eNO) levels is thought to be a useful marker of asthmatic airway inflammation, and may therefore serve as an additional diagnostic tool that can be measured noninvasively in adults, children, and even infants. (6-8) eNO levels are elevated in asthmatic children compared to healthy children and are reduced after antiinflammatory therapy. (7,9-11)
Measurements of lung function form an important part of the clinical assessment and management of older children and adults with asthma. But while lung function tests can easily be performed in adults and children > 6 years of age, the routine methods are difficult to perform in younger children due to their insufficient cooperation. (12) In preschool children, only a small number of techniques are suitable. While aware of the limitations of these techniques, we measured airway resistance using the interrupter technique (Rint), because this technique requires only little cooperation and can be performed even in very young children. (13) Rint measurements have been shown to be a reliable method for assessing lung function and bronchodilator response in this age group. (14)
To a certain extent, the lack of response to inhaled antiinflammatory treatment in young children may be explained by the difficulty of drug administration. Studies have shown that in children in this age group only a small amount of the inhaled drug is deposited in the lungs due to insufficient cooperation. (15) Montelukast (Singulair; MSD; Glattbrugg, Switzerland), an orally available leukotriene receptor antagonist (LTBA), may therefore be a good alternative for anti-inflammatory treatment in young children. Cysteinyl leukotrienes play an important role in the cascade of asthmatic airway inflammation, and lead to increased mucus secretion, epithelial cell damage, smooth muscle proliferation and constriction, edema, and the influx of inflammatory cells. (16) As an LTRA, montelukast blocks the effects of cysteinyl leukotrienes, and therefore has a beneficial effect on airway inflammation and bronchoconstriction. (17) Montelukast has been shown to be a potential antiinflammatory drug in school children with asthma, and may be advantageous over inhaled steroids due to its oral, and therefore easy, administration and calculable drug deposition, which cannot be obtained with inhalation therapy, particularly in young children. (18) We aimed to investigate the therapeutic effect of montelukast on eNO, airway resistance, and bronchodilator responsiveness in a well-defined group of preschool children with allergic asthma.
MATERIALS AND METHODS
Study Design
Children who were referred to our outpatient clinic due to recurrent wheeze over a period of 6 months, and who were symptomatic at the time of referral and had a positive parental history" of asthma, were allocated to a run-in period if their eNO level was [greater than or equal to] 15 parts per billion (ppb) and underwent a blood test (ie, the radioallergosorbent test [RAST]) to detect either food or inhaled allergens as well as a lung function measure (ie, Rint) before the run-in period began (visit 1). Therapy with inhaled bronchodilators had been stopped at least 24 h prior to visit 1.
After a 1-week run in period during which subjects received only on-demand bronchodilator therapy, 30 preschool children (19 girls; mean age, 41.2 months; age range, 24 to 60 months) with verified atopy (defined as RAST class > 1 for at least one of the most frequent allergens) received diagnoses of preschool asthma and were recruited for the study. Again, eNO levels together with lung function and bronchodilator responsiveness were measured (visit 2) and treatment with montelukast (4 mg once daily) was commenced in all patients. Adherence to therapy was controlled by parents' reports on the daily use of montelukast and by counting returned tablets, eNO level, lung function, and bronchodilator responsiveness were measured again after 4 weeks of treatment (visit 3). The study was approved by the local ethics committee, and informed consent was obtained for all patients.
Lung Function
Rint was measured (MicroRint; MieroMedieal Ltd; Rochester, UK), and all tests were performed by two trained lung function technicians to minimize the interobserver variability, as shown previously. (19) During tidal breathing, interruptions were randomly performed at the beginning of exhalation, while supporting the cheeks and with the head slightly raised. If possible, children performed the test via a mouthpiece, if not via a face mask. Ten minutes after the administration of three puffs (750 [mu]g) terbutaline sulfate from a pressurised metered-dose inhaler (Bricanyl; AstraZeneca; Zug, Switzerland) through a spacer (Nebunette; AstraZeneca), measurements were repeated to detect bronchodilator responsiveness. Technically invalid values were excluded, and the median of a minimum of six acceptable measurements with an intrasubject coefficient of variation of < 20% was reported.
eNO Measurements
eNO was measured using the single-breath positive expiratory technique according to American Thoracic Society recommendations or, if not possible due to patient cooperation (eight patients), using an offline-reservoir technique that has been described previously. (9) Therefore, infants were seated on the legs of the mother and breathed through a face mask. The face mask was attached to a two-way valve that allowed the inspiration of nitric oxide (NO)-free air from a reservoir to prevent contamination with ambient air. After 10 breaths of NO-free air, a reusable 750-mL collection bag (Quintron; Milwaukee, WI) was attached to the expiratory side of the valve and five breaths were collected. The NO concentration in the bags was analyzed within 15 min of collection using a fast-response chemoluminescence analyzer (CLD 77 AM; Eco Medics; Durnten, Switzerland) at a sampling flow of 100 mL/min.
Online eNO measurement by the single-breath positive expiratory technique was performed according to American Thoracic Society recommendations in 22 children. (20) Forced eNO was analyzed using a chemiluminescence analyzer (CLD 77; Eco Medics) with a sampling rate of 100 Hz. Children were instructed to perform an exhalation against an expiratory resistance (0.5 to 2.0 kPa) to achieve a constant expiratory flow rate of 50 mL/s, which was supported by an visual feedback system. Measurements were repeated, and the average of three technically acceptable measurements was recorded.
Statistical Analysis
Descriptive statistical analyses were performed. Values for eNO and lung function parameters were normally distributed and were expressed as the mean (SD). Repeated measurements of analysis of variance were performed using contrast vectors for specific conditions or time points. All results were obtained using a statistical software package (SYSTAT 10 Software; SPSS Inc; Chicago, IL).
RESULTS
From 55 preschool children with recurrent wheeze and a parental history of atopy, 9 had to be excluded due to their inability to perform valid Rint measurements. Forty-six children were allocated to the run-in period, but only 30 fulfilled the inclusion criteria, including positive RAST test result, and were included in the study.
The mean eNO levels at visits 1, 2, and 3 were 31.4 ppb (SD, 13.9 ppb), 33.1 ppb (SD, 12.0 ppb), and 11.6 ppb (SD, 9.5 ppb), respectively. The mean Rint values at visit 1, 2, and 3 were 1.26 kPa/L/s (SD, 0.27 kPa/L/s), 1.28 kPa/L/s (SD, 0.23 kPa/L/s), and 1.15 kPa/L/s (SD, 0.26 kPa/L/s), respectively.
There was no significant difference in eNO measurements, airway resistance, as well as bronchodilator responsiveness between visits 1 and 2 (p = 0.56, p = 0.8, and p = 0.75, respectively). There was a significant decrease in eNO levels after 4 weeks of treatment with montelukast (p < 0.0005) [Fig 1]. The reduction in airway resistance (Rint) after 4 weeks of montelukast therapy was significant (p = 0.01) [Fig 2].
[FIGURES 1-2 OMITTED]
The mean bronchial dilating effect after the administration of terbutaline sulfate was 13.4% (SD, 5.8%), 13.2% (SD, 6.8%), and 13.3% (SD, 7.0%), respectively, after visits 1, 2, and 3, and the differences were not significant different between visit 1/visit 2 and visit 3 (p = 0.75 and p = 0.47, respectively) [Fig 3].
[FIGURE 3 OMITTED]
DISCUSSION
In the present study, we investigated the effect of montelukast on levels of eNO and pulmonary function in preschool children with allergic asthma. We included children with chronic wheeze, raised eNO levels, and parental atopy to ensure a degree of homogeneity with regard to phenotype and to maximize the likelihood of persistent airway inflammation being present. Levels of eNO and airway resistance were significantly lower after 4 weeks of treatment with montelukast.
Current guidelines recommend the use of inhaled steroids as the first-choice treatment for mild persistent asthma in children < 5 years of age. (21) The effectiveness of montelukast therapy has been investigated in some previous studies. Montelukast has been shown to reduce bronchial hyperresponsivehess, to protect against exercise-induced and allergen-induced bronchoconstriction, to reduce airway inflammation, and, as an add-on therapy, to improve asthma control. (22-25)
In adults and school-aged children, montelukast therapy has been shown to cause a reduction of eNO levels, reflecting its antiinflammatory potency. (10,11,24,26,27) The primary outcomes of most studies investigating therapy with montelukast in young asthmatic children were clinical findings, respiratory symptoms, and the need for reliever medication, and only a few studies have included objective parameters such as lung function parameters and markers of inflammation. Knorr et al (28) found a significant decrease in blood eosinophils during (12) weeks of therapy and in the need for reliever medication, as well as an improvement of symptoms and in the percentage of symptom-free days in preschool children. Bisgaard et al (10) investigated the effect of montelukast therapy on levels of eNO in asthmatic school children and found a rapid fall in eNO levels, independent of concurrent steroid treatment.
Our data from objective measurements suggest that airway inflammation is present in a well-defined subgroup of asthmatic preschool children with raised eNO levels. The decline in eNO levels may affirm the antiinflammatory effect of montelukast in these young asthmatic children. However, this conclusion must be considered cautiously since the relationship between eNO levels and airway inflammation has not been fully resolved, and the lack of a control group may limit the conclusions drawn from the present study. (29,30)
The consistent bronchodilator effect at all three visits is in agreement with the bronchodilator effect found in healthy infants and children. (14) Despite ongoing airway inflammation, the children included in our study were nearly symptom-free at the time of the investigation. Levels of eNO and airway resistance were significantly lower after 4 weeks of treatment with montelukast. This was in contrast to measurements of bronchodilator responsiveness, in which no changes were observed. Despite the lack of improvement in bronchodilator responsiveness in this study, it is important to note that our observations agree with those from a study by Kharitonov et al, (31) in which measurements of eNO in adult asthmatic patients were shown to be more sensitive to changes in inhaled steroid doses than measurements of spirometry and peak flow, the need for rescue medication, or the results of diary recordings. It has been suggested that decreased airway inflammation and improvement of airway obstruction will occur before noticeable changes in airway hyperreactivity. (32) Based on this explanation, changes in bronchodilator responsiveness may have been difficult to detect in the present study because of the relatively short treatment period.
As demonstrated in Figure 2, a decline in airway resistance can be demonstrated in most patients, but not in all, not even in our well-defined subgroup of young asthmatic children with proven atopy, a parental history for asthma, and elevated levels of eNO. Several studies (33,34) have demonstrated a heterogeneity of patient response to different asthma medications, but the question of why some asthmatic patients respond to antiinflammatory therapy while others do not has not yet been answered. It is a point of discussion of whether the individual genetic background plays an important role in the variability of the clinical response to LTRAs. The biosynthesis of cysteinyl leukotrienes has been extensively investigated, and, after localization of the gene for leukotriene [C.sub.4], a single-nucleotide polymorphism was found. (35) This polymorphism is an example of a genetic variant affecting protein expression and may predispose the patient to a highly leukotriene-dependent form of asthma. The results of preliminary studies seem to support the hypothesis that a benefit resulting from therapy with LTRAs may depend on the patients genetic background. (36-38)
In summary, our study has demonstrated that montelukast decreased levels of eNO and airway resistance in preschool children with allergic asthma and, hence, that an antiinflammatory treatment with montelukast over a short time period can be beneficial. We conclude that montelukast may be a good alternative first-line antiinflammatory treatment in preschool children with allergic asthma, in general, and in children who display a lack of cooperation with inhaled therapy, in particular. Whether this finding, as well as the long-term benefit found for montelukast therapy, applies to younger children and/or children with other etiologies of wheeze still has to be investigated.
* From the University Children's Hospital Zurich (Drs. Straub, Minocchieri, Moeller, and Wildhaber), Swiss Paediatric Respiratory Research Group, Zurich, Switzerland; and University of Homburg (Dr. Hamacher), Homburg, Germany.
REFERENCES
(1) McKean M, Ducharme F. Inhaled steroids for episodic viral wheeze of childhood. Cochrane Database Syst Rev (database online). Issue 2, 2000
(2) Roorda RJ, Mezei G, Bisgaard H, et al. Response of preschool children with asthma symptoms to fluticasone propionate. J Allergy Clin Immunol 2001; 108:540-546
(3) Volovitz B, Bentur L, Finkelstein Y, et al. Effectiveness and safety of inhaled corticosteroids in controlling acute asthma attacks in children who were treated in the emergency department: a controlled comparative study with oral prednisolone. J Allergy Clin Immunol 1998; 102:605-609
(4) Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life: the Group Health Medical Associates. N Engl J Med 1995; 332:133-138
(5) Martinez FD. Present and future treatment of asthma in infants and young children. J Allergy Clin Immunol 1999; 104:169 -174
(6) Kharitonov SA, Yates D, Springall DR, et al. Exhaled nitric oxide is increased in asthma. Chest 1995; 107:156S-157S
(7) Byrnes CA, Dinarevie S, Shinebourne EA, et al. Exhaled nitric oxide measurements in normal and asthmatic children. Pediatr Pulmonol 1997; 24:312-318
(8) Wildhaber JH, Hall GL, Stick SM. Measurements of exhaled nitric oxide with the single-breath technique and positive expiratory pressure in infants. Am J Respir Crit Care Med 1999; 159:74-78
(9) Baraldi E, Dario C, Ongaro R, et al. Exhaled nitric oxide concentrations during treatment of wheezing exacerbation in infants and young children. Am J Respir Crit Care Med 1999; 159:1284-1288
(10) Bisgaard H, Loland L, Oj JA. NO in exhaled air of asthmatic children is reduced by the leukotriene receptor antagonist montelukast. Am J Respir Crit Care Med 1999; 160:1227-1231
(11) Wildhaber JH, Moeller A, Hall GH, et al. Levels of exhaled nitric oxide in recurrently wheezy infants are decreased following inhaled steroid therapy. Schweiz Med Wochenschr 2000; 130:529-534
(12) Kanengiser S, Dozor AJ. Forced expiratory maneuvers in children aged 3 to 5 years. Pediatr Pulmonol 1994; 18:144-149
(13) Phagoo SB, Wilson NM, Silverman M. Evaluation of a new interrupter device for measuring bronchial responsiveness and the response to bronchodilator in 3 year old children. Eur Respir J 1996; 9:1374-1380
(14) Beydon N, Amsallem F, Bellet M, et al. Pre/postbronchodilator interrupter resistance values in healthy young children. Am J Respir Crit Care Med 2002; 165:1388-1394
(15) Salmon B, Wilson NM, Silverman M. How much aerosol reaches the lungs of wheezy infants and toddlers? Arch Dis Child 1990; 65:401-403
(16) Hay DW, Torphy TJ, Undem BJ. Cysteinyl leukotrienes in asthma: old mediators up to new tricks. Trends Pharmacol Sci 1995; 16:304-309
(17) Bisgaard H. Pathophysiology of the cysteinyl leukotrienes and effects of leukotriene receptor antagonists in asthma. Allergy 2001; 56:7-11
(18) Narayanan S, Edelman JM, Berger ML, et al. Asthma control and patient satisfaction among early pediatric users of montelukast. J Asthma 2002; 39:757-765
(19) Klug B, Nielsen KG, Bisgaard H. Observer variability of lung function measurements in 2-6-yr-old children. Eur Respir J 2000; 16:472-475
(20) Recommendations for standardized procedures for the online and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children-1999. Am J Respir Crit Care Med 1999; 160:2104-2117
(21) Global Initiative for Asthma. GINA guidelines: global strategy for asthma management and prevention revised (2002). Bethesda, MD: National Institutes of Health, 2002; publication No. 02-3659
(22) Berkman N, Avital A, Bardach E, et al. The effect of montelukast on bronchial provocation tests and exhaled nitric oxide levels in asthmatic patients. Isr Med Assoc J 2003; 5:778-781
(23) Kemp JP, Dockhorn RJ, Shapiro GG, et al. Montelukast once daily inhibits exercise-induced bronchoconstriction in 6- to 14-year-old children with asthma. J Pediatr 1998; 133:424-428
(24) Minoguchi K, Kohno Y, Minoguchi H, et al. Reduction of eosinophilic inflammation in the airways of patients with asthma using montelukast. Chest 2002; 121:732-738
(25) Price DB, Hernandez D, Magyar P, et al. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax 2003; 58:211-216
(26) Bratton DL, Lanz MJ, Miyazawa N, et al. Exhaled nitric oxide before and after montelukast sodium therapy in school-age children with chronic asthma: a preliminary study. Pediatr Pulmonol 1999; 28:402-407
(27) Stehnach I, Jerzynska J, Kuna P. A randomized, double-blind trial of the effect of treatment with montelukast on bronchial hyperresponsiveness and serum eosinophilic cationic protein (ECP), soluble interleukin 2 receptor (sIL-2R), IL-4, and soluble intercellular adhesion molecule 1 (sICAM-1) in children with asthma. J Allergy Clin Immunol 2002; 109:257-263
(28) Knorr B, Franchi LM, Bisgaard H, et al. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics 2001; 108:E48
(29) Lim S, Jatakanon A, John M, et al. Effect of inhaled budesonide on lung function and airway inflammation: assessment by various inflammatory markers in mild asthma. Am J Respir Crit Care Med 1999; 159:22-30
(30) Berlyne GS, Parameswaran K, Kamada D, et al. A comparison of exhaled nitric oxide and induced sputum as markers of airway inflammation. J Allergy Clin Immunol 2000; 106:638-644
(31) Kharitonov SA, Yates DH, Barnes PJ. Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. Am J Respir Crit Care Med 1996; 1.53:454-457
(32) Bates CA, Silkoff PE. Exhaled nitric oxide in asthma: from bench to bedside. J Allergy Clin Immunol 2003; 111:256-262
(33) Szefler SJ, Martin RJ, King TS, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol 2002; 109:410-418
(.3)4 Malmstrom K, Rodriguez-Gomez G, Guerra J, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma: a randomized, controlled trial: Montelukast/ Beclomethasone Study Group. Ann Intern Med 1999; 130: 487-495
(35) Sanak M, Simon HU, Szczeklik A. Leukotriene C4 synthase promoter polymorphism and risk of aspirin-induced asthma. Lancet 1997; 350:1599-1600
(36) Sampson AP, Siddiqui S, Buchanan D, et al. Variant LTC(4) synthase allele modifies cysteinyl leukotriene synthesis in eosinophils and predicts clinical response to zafirlukast. Thorax 2000; 55:S28-S31
(37) Mastalerz L, Nizankowska E, Sanak M, et al. Clinical and genetic features underlying the response of patients with bronchial asthma to treatment with a leukotriene receptor antagonist. Eur J Clin Invest 2002; 32:949-955
(38) Whelan GJ, Blake K, Kissoon N, et al. Effect of montelukast on time-course of exhaled nitric oxide in asthma: influence of LTC4 synthase A(-444)C polymorphism. Pediatr Pulmonol 2003; 36:413-420
This study was funded by a Medical School Grant (MSD). Manuscript received March 24, 2004; revision accepted August 11, 2004.
Correspondence to: Daniel A. Straub, MD, Division of Respiratory Medicine, University Children's Hospital, Steinwiesstrasse 75, CH-8032 Zurich, Switzerland; e-mail: daniel.straub@ kispi.unizh.ch
COPYRIGHT 2005 American College of Chest Physicians
COPYRIGHT 2005 Gale Group