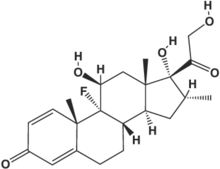
Abbreviation: HASM = human airway smooth muscle
We examined the ability of the corticosteroid dexamethasone to inhibit short-term (1 h) and long-term (24 h) [[beta].sub.2]-adrenergic desensitization in cultured human airway smooth muscle (HASM) cells. In cells from 19 individuals studied on 47 experimental days, pretreatment with isoproterenol at [10.sup.-7] mol/L for 1 h or 24 h caused approximately 21% and 77% decreases in the ability of subsequent isoproterenol ([10.sup.-6] mol/L) stimulation to reduce HASM cell stiffness as measured by magnetic twisting cytometry, similar to results we have previously reported. (1) We examined the ability of dexamethasone ([10.sup.-6] mol/L) administered 6 h or 48 h prior to isoproterenol pretreatment, and stratified our results by presence or absence of the Arg19 allele of the [[beta].sub.2]AR upstream peptide, located in the 5'-leader cistron of the [[beta].sub.2]AR gene. This polymorphism has been associated with decreased [[beta].sub.2]AR expression in HASM cells. In cells containing at least one Arg19 allele, dexamethasone did not significantly reverse short-term or long-term desensitization, but in cells without the Arg19 allele, dexamethasone significantly reversed short-term and long-term desensitization (35% and 31%, respectively; p < 0.05 for each). Our results suggest that the Arg19 allele, or other polymorphisms of the promoter of the [[beta].sub.2]AR gene that are in linkage disequilibrium, may affect the ability of dexamethasone to upregulate [[beta].sub.2]AR expression in HASM cells. In addition, our results suggest that [[beta].sub.2]AR genotype may be predictive of corticosteroid responsiveness in individuals with asthma.
REFERENCE
(1) Moore PE, Laporte PD, Abraham JH, et al. Polymorphism of the [[beta].sub.2]-adrenergic receptor gene and desensitization in human airway smooth muscle. Am J Respir Crit Care Med 2000; 162:2117-2124
* From the Departments of Pediatrics and Pharmacology (Dr. Moore and Mr. Calder), Vanderbilt University School of Medicine, Nashville, TN; Physiology Program (Drs. Silverman and Shore), Harvard School of Public Health, Boston, MA; and Department of Medicine (Dr. Panettieri), University of Pennsylvania, Philadelphia, PA.
This abstract is supported by National Institutes of Health grants HL04395, HL56383, HL33009, and HL67663, and the American Lung Association.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (e-mail: permissions@chestnet.org).
Correspondence to: Paul E. Moore, MD, Division of Pediatric Pulmonary Medicine, Vanderbilt University School of Medicine, 1161 21st Ave South, S0119 MCN, Nashville, TN 37232-2586
COPYRIGHT 2003 American College of Chest Physicians
COPYRIGHT 2003 Gale Group