Abstract
The objective of this study was to develop a rapid-release diazepam suppository. Four kinds of diazepam suppositories were formulated: (1) a conventional suppository with Witepsol H-15 as a base, (2) a conventional suppository with mixed polyethylene glycols as a base, (3) a hollow-type suppository with Witepsol H-15 as a base that contained diazepam solution in its cavity and (4) a hollow-type suppository with Witepsol H-15 as a base that contained diazepam powder in its cavity. The result of the differential scanning calorimetry thermograms indicated that diazepam dissolved in Witepsol base had strong affinity to the lipophilic base while diazepam dispersed in polyethylene glycol base was still in the crystalline form. The release study of prepared suppositories showed that the release of diazepam from suppository 1 and suppository 4 was very slow. The diazepam released from suppository 2 after 5 minutes was about 30%. The diazepam released from suppository 3 after 5 minutes was about 85%, which was significantly faster than that of the other three formulations (1, 2 and 4). The hollow-type suppository that contained diazepam solution was found to be the most effective rapid-release formulation.
Introduction
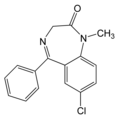
Diazepam is a long-acting benzodiazepine with anticonvulsant, sedative and anxiolytic properties.1 It is rapidly absorbed, enters the brain quickly and interrupts seizures within S to 15 minutes. Generally, intravenous diazepam is the drug of choice for the emergency treatment of convulsion status epilepticus; however, it can only be administered in a hospital setting and it is not an ideal medication for emergent treatment in the community by nonmedical personnel. Rectal formulations not only enable caregivers (parents, family) to treat uncontrolled seizures immediately but may also be administered by a physician when intravenous access is not possible. Because diazepam rectal formulations (rectal solutions, rectal gels) are rapidly absorbed, peak plasma concentrations are attained within 20 minutes.
However, rectal diazepam solutions have not been used widely because they tend to leak out of the rectum, which can lead to inaccurate dosing and treatment failure.2
Diazepam in a conventional suppository is well absorbed, but absorption is slow because of the slow release of the drug from the suppository base. Studies3-4 have been conducted in an effort to determine how to increase the diazepam release rate from a suppository. Hollow-type suppositories have some advantages over conventional suppositories: they can carry either powdered or solution forms of drugs and they eliminate the effect of the heating process on the nature of the drug during the preparation of the suppository. Watanabe et al5 demonstrated that drug was released more rapidly and absorbed more efficiently from a hollow-type suppository than from a conventional suppository.
The objective of the present research was to develop the most effective rapid-release diazepam suppository by incorporating the diazepam, in either crystalline form or aqueous solution, into the cavity of a hollow-type suppository. In this study we compared the release of diazepam from hollow-type suppositories with that from conventional suppositories.
Materials and Methods
Materials
Diazepam (Lot 300304) was donated by Atlantic Pharmaceutical Industry (Bangkok, Thailand). Witepsol H-15 (Lot 905708) was donated by Condea Chemie GMbH (Witten, Germany). Polyethylene glycol (PEG) 400 (Lot 99-1548) and PEG 4000 (Lot 45-1275) were provided by BASF (Bangkok). All other chemicals used were reagent grade.
Methods
Diazepam Suppository Preparations
Conventional suppositories were prepared by the fusion method with Witepsol H-15 or PEG base (PEG 400 and PEG 4000 in the ratio of 1:1). The bases were melted using a water bath, diazepam powder was added with stirring until a homogeneous mixture was produced, the mixture was poured into the metal suppository mold and then the mixture was cooled. Hollow-type suppositories (Figure 1) with Witepsol H-15 as the base were prepared by the method reported by Watanabe et al.5 The method is summarized as follows: Witepsol H-15 was melted at approximately 45°C, poured into a suppository mold equipped with a cylindrical tube in the center and allowed to stand for 2 hours at room temperature to solidify. After construction of a hollow cavity in the solidified Witepsol H-15, either a diazepam powder (prepared by mixing diazepam and lactose in a ratio of 1:9), or a 400-µL diazepam solution (prepared by mixing diazepam powder in an aqueous solution of 4% Tween 80 and propylene glycol) was added to each cavity. The opening at the back part of the suppository was sealed with the melted base. Each suppository contained an amount of solution or powder equivalent to 10 mg of diazepam.
Differential Scanning Calorimetry
The thermal properties of diazepam, Witepsol H-15, PEG base and diazepam suppositories were studied on a differential scanning calorimeter (DSC) (Perkin-Elmer DSC7, Norwalk, Connecticut). Samples (2 to 8 mg) were accurately weighed and heated in closed aluminum crimp cells at the rate of 10°C/minute under nitrogen purge with a flow rate of 35 mL/minute over a temperature range of 20°C to 150°C.
Evaluation of Physical Properties of Suppositories
The prepared suppositories were evaluated for disintegration according to a method described in the British Pharmacopoeia (BP).6 Each determination was carried out in triplicate.
In Vitro Drug-Release Studies
The release rates of diazepam from conventional and hollow-type suppositories were determined. A rotating basket dissolution apparatus was used at 50 ± 1 rpm at a constant temperature of 37°C ± 0.5°C. The medium used was 500 mL of phosphate buffer solution, pH 7.4. At appropriate time intervals, 4-mL samples were withdrawn and the amount of diazepam was determined by ultraviolet spectrophotometry (Spectronic Genesys 5, Milton Roy, Rochester, New York) at 232 nm using appropriate blank solutions. Diazepam concentration was calculated and expressed as percentage of drug released from the mean of six determinations.
Results and Discussion
The disintegration time for the prepared suppositories 1, 2, 3 and 4 was 9, 2R, 8 and 9 minutes, respectively. According to the BP requirement, disintegration occurs in not more than 30 minutes for lipophilic-based suppositories and in not more than 60 minutes for water-soluble suppositories. Therefore, all suppositories were found to satisfy the BP requirement for disintegration.
Because of its good resistance to oxidation and its lack of polymorphism problems, this study used Witepsol H-15 mixtures of synthetic of triglycerides as a lipophilic base instead of theobroma oil. Figure 2 shows the DSC thermograms of diazepam, Witepsol H-15, PEG base and the diazepam suppositories. The thermogram of diazepam showed a single sharp endothermic peak at the melting point of 139°C. Witepsol H-15 showed an endothermic peak at 36°C, and the PEG base showed endothermic peaks at 43°C and 50°C, corresponding to the melting points of the base. The thermogram of the diazepam suppository in Witepsol H-15 showed only one endothermic peak at 38°C with the endothermic peak of diazepam absent, suggesting that diazepam is completely soluble in the Witepsol base.7 The thermogram of the diazepam suppository in PEG base showed both the endothermic peaks of this base at 43°C and 51°C and the endothermic peak of diazepam shifted slightly to a lower temperature (approximately 137°C), suggesting that the diazepam that was dispersed in PEG base was still in the crystalline form.
The percentage of diazepam released from the conventional and the hollow-type suppositories is shown in Figure 3. This study did not show the result of the PEG hollow-type suppository containing diazepam powder because the release of diazepam from a PEG hollow-type suppository is very slow, and lower than from the Witepsol H-15 base hollow-type suppository that contained diazepam powder. The rate of release of diazepam from the conventional suppository with PEG as the hydrophilic base was about 30% after 5 minutes and exhibited complete release after 30 minutes. The rate of release of diazepam from the conventional suppository with Witepsol H-IS as the lipophilic base was only 5% after 5 minutes and 20% after 120 minutes. The faster release rate of diazepam from the PEG base may be due to both the low affinity of the drug for the base and the water solubility of the base, which allows the drug to be released by both diffusion and erosion mechanisms.8
The slower release rate observed in the conventional suppository using Witepsol H-15 as the base is probably due to the strong affinity of the drug for the lipophilic base, which results in hindered migration of diazepam molecules in the dissolution medium.9 Therefore, Witepsol H-15 is not suitable for diazepam rapid-release conventional suppositories. The rate of release of diazepam from the hollow-type suppositories that contained the powder was very slow. This may be because the drug was trapped in the melted base. The release rate of diazepam from the hollow-type suppositories containing the diazepam solution in their cavity was 85% after 5 minutes, which was significantly faster than the rate of release from the conventional suppositories that had either PEG or Witepsol as their base. This may be because of the rapid melting of Witepsol H-15, which allowed the rapid release of the aqueous solution of diazepam.
Conclusion
The rate of release of diazepam from various suppository formulations was studied. The hollow-type suppository that contained diazepam solution with Witepsol H-15 as the base exhibited the fastest rate of release of diazepam. Both the Witepsol H-15-base hollow-type suppository that contained diazepam powder and the Witepsol H-15-base conventional suppository gave slower release rates. The Witepsol H-15-base hollow-type suppository containing diazepam solution was found to be the most effective rapid-release formulation in this study and may be useful as a rectal dosage form. However, further studies are required to demonstrate the stability of diazepam from this suppository and the rectal absorption of the drug in the human body.
References
1. Sweetman SC, ed. MARTINDALE: The Complete Drug Reference. 33rd ed. London: The Pharmaceutical Press; 2002: 680.
2. Fitzgerald BJ, Okos AJ, Miller JW. Treatment of out-of-hospital status epilepticus with diazepam rectal gel. Seizure 2003; 12(1): 52-55.
3. Deshmukh AA, Thwaite S. In vitro release of diazepam from conventional and double-layer polyethylene glycol suppositories. Drug Dev Ind Pharm 1989; 15: 1289-1307.
4. Regdon G, Bacskay I, Kata M et al. Formulation of diazepam containing rectal suppositories and experiences of their biopharmaceutical study. Pharmazie 1994; 49(5): 346-349.
5. Watanabe Y, Matsumoto Y, Baba K et al. Pharmaceutical evaluation of hollow type suppositories. IV. Improvement of bioavailability of pro pranolol in rabbits after rectal administration. J Pharmacobiodyn 1986; 9(6): 526-531.
6. [No author listed.] British Pharmacopoeia. London: The Stationary Office; 2001 : A235.
7. Mura P, Manderioli A, Bramanti G et al. The properties of solid dispersions of nap roxen in various polyethylene glycols. Drug Dev Ind Pharm 1996; 22(9 & 10): 909-916.
8. Hosny EA, Abdel-Hady SS, EI-Tahir KEH. Formulation, in-vitro release and ex-vivo spasmolytic effects of mebeverine hydrochloride suppositories containing polycarbophil or polysorbate 80. Int J Pharm 1996; 142: 163-168.
9. Tanaka M, Kuwahara E, Takahashi M et al. Enhanced rectal absorption of amphotericin B lyophilized with glycyrrhizinate in rabbits. Biol Pharm Bull 1998; 21(8): 853-857.
N. Kaewnopparat, MSPharm
S. Kaewnopparat, PhD, BioPharm
W. Rojanarat, BSPharm
S. Ingkatawornwong, MSPharm
Prince of Songkla University Hat-Yai, Songkla Thailand
Address correspondence to: Nattha Kaewnopparat, MSPharm, Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Hat-Yai, Songkla 90112 Thailand. E-mail: knattha@ratree.psu.ac.th
Copyright International Journal of Pharmaceutical Compounding Jul/Aug 2004
Provided by ProQuest Information and Learning Company. All rights Reserved