For 100 mL
Diazepam 500 mg
Propylene glycol 40 mL
Alcohol 10 mL
Benzoic acid 3.5 g
Benzyl alcohol 1.5 g
Sodium hydroxide 20% solution qs
Sterile water for injection qs 100 mL
METHOD OF PREPARATION
Note: This preparation should be prepared in a laminar flow hood in a cleanroom (or via isolation barrier technology) by a validated aseptic compounding pharmacist using strict aseptic technique.
1. Calculate the required quantity of each ingredient for the total amount to be prepared.
2. Accurately weigh and/or measure each ingredient.
3. Mix the propylene glycol, alcohol, and benzyl alcohol.
4. Dissolve the diazepam and benzoic acid in the solution from Step 3.
5. Slowly add sterile water for injection to a volume of 75 to 80 mL.
6. Add the sodium hydroxide solution (dropwise) to adjust the pH to the range of 6.2 to 6.9.
7. Add sufficient sterile water for injection to volume and mix well.
8. Filter through an appropriate sterile 0.2-gm filter into sterile vials.
9. Package and label. PACKAGING
Package in sterile glass vials.
LABELING
For professional use.
STABILITY
A beyond-use date of up to 6 months can be used for this preparation.1
USE
Diazepam is a benzodiazepine that has anticonvulsant, anxiolytic, sedative, muscle-relaxant, and amnesic properties.2-4
QUALITY CONTROL
Quality-control assessment can include weight and/or volume, physical observation, pH, specific gravity, osmolality, assay, color, clarity, particulate matter, sterility, and pyrogenicity testing.5,6
DISCUSSION
Diazepam is a long-acting benzodiazepine, which has many applications that may be used in the event of terrorism. It can be administered orally, parenterally, or rectally. If diluting this injection, care should be observed due to the cosolvent system used to solubilize the diazepam. The benzoic acid:sodium benzoate buffer system is formed in situ upon the addition of the sodium hydroxide to the solution.
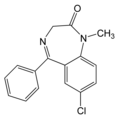
Diazepam (C^sub 16^H^sub 13^C1N^sub 2^O, MW 284.75, Valium) occurs as an off-white to yellow, practically odorless, crystalline powder. It is practically insoluble in water (1 g in 333 mL water) but is soluble in alcohol (1 g in 16 mL). It melts between 131deg C and 135deg C.1-3
Propylene glycol (C^sub 3^H^sub 8^O^sub 2^, MW 76.09) occurs as a clear, colorless, viscous, practically odorless liquid with a sweet taste, somewhat resembling glycerin. It has a specific gravity of 1.038 g/mL and is miscible with acetone, chloroform, 95% ethanol, glycerin, and water.7
Alcohol (C^sub 2^H^sub 5^OH, MW 46, ethyl alcohol, ethanol, grain alcohol) is a clear, colorless, mobile, and volatile liquid with a slight, characteristic odor and a burning taste. It is miscible with chloroform, glycerin, and water, and its solutions may be sterilized by autoclaving or by filtration.8
Benzoic acid (C^sub 7^H^sub 6^O^sub 2^, MW 122.12) occurs as white crystals, scales, or needles, with a slight odor. It is somewhat volatile at warmer temperatures, is slightly soluble in water, and is freely soluble in alcohol.1
Benzyl alcohol (C^sub 7^H^sub 8^O, MW 108.14) is an antimicrobial preservative, disinfectant, and solvent. It is a clear, colorless, oily liquid that has a faint, aromatic odor and a sharp, burning taste. It is soluble 1g in 25 mL of water at 25degC and is miscible with ethanol.9
Sodium hydroxide (NaOH, MW 40.00, caustic soda, soda lye) occurs as dry, very deliquescent, white or almost white sticks, pellets, or fused masses, which are hard and brittle. It is soluble 1 g in 1 mL of water and is freely soluble in alcohol.' Sterile water for injection is water that has been sterilized and suitably packaged; it contains no added substances.1,10
References
1. US Pharmacopeial Convention, Inc. United States Pharmacopeia 25/National Formulary 20. Rockville, MD:US Pharmacopeial Convention, Inc; 2001:545-547,2374, 1753, 2053-2057, 2367.
2. Reynolds JEF, ed. MARTINDALE: The Extra Pharmacopeia. 30th ed. London:The Pharmaceutical Press; 1993:584-585, 1415.
3. McEvoy GK. AHFS Drug Information-2002. Bethesda, MD:American Society of Health-System Pharmacists; 2002:2389-2392.
4. [No author listed.] Physicians' Desk Reference. 56th ed. Montvale, NJ:Medical Economics Company; 2002:3026-3027.
5. Allen LV Jr. Standard operating procedure for particulate testing for sterile products. IJPC 1998;2:78.
6. Allen LV Jr. Standard operating procedure: Quality assessment for injectable solutions. IJPC 1999;3:406-407.
7. Dandiker Y. Propylene glycol. In: Kibbe A, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC:American Pharmaceutical Association; 2000:442-444.
8. Weller PJ. Alcohol. In: Kibbe A, ed. Handbook of Pharmaceutical Exciplents. 3rd ed. Washington, DC:American Pharmaceutical Association; 2000:7-9.
9. Brunson EL. Benzyl alcohol. In: Kibbe A, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC:American Pharmaceutical Association; 2000:41-43.
10. Horry JM, Nash RA. Water. In: Kibbe AH, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC:American Pharmaceutical Association; 2000:546-549.
Copyright International Journal of Pharmaceutical Compounding Mar/Apr 2003
Provided by ProQuest Information and Learning Company. All rights Reserved