Abstract
Picaridin is a new insect repellent that is comparable in effect and less irritating than diethyl toluamide (deet). Its activity and effects are reviewed in this article.
**********
Discussion
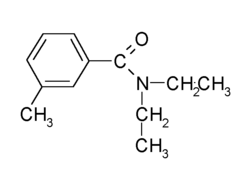
Picaridin, a piperidine derivate, is a new insect repellent. Its chemical formula is C12H23NO3 (Figure 1). Its International Union of Practical and Applied Chemistry (IUPAC) name is (RS)-sec-butyl 2-(2-hydroxyethyl)piperidine-1-carboxylate. The name "picaridin" is approved by the World Health Organization, but there is no International Organization for Standardization common name for this substance. Picaridin's other names include KBR3023. Bayrepel. Hepidanin, and Autan Repel (1). It is effective against a range of insects (2) (Table 1).
[FIGURE 1 OMITTED]
Picaridin is available in Europe and Australia, and will soon be sold in North America. It is sold in Australia in spray and lotion forms with 92.8 gm/L picaridin as the active ingredient. A formulation with 192 grams/liter is termed Autan Repel Army 20.
As stated above, picaridin is not yet available in the United States. It was registered as a "reduced-risk" chemical by the Environmental Protection Agency (EPA) in 2000-2001 (3). Bayer Corporation has received full, unconditional registration for KBR 3023 with the active ingredient picaridin (4). Products that contain KBR that are currently planned to be sold in the United States include a 5% cream, a 5% non-aerosol pump spray, and a 10% aerosol spray (5). They will be sold by S.G. Johnson and Sons and will join 200 different formulations of insect repellants (most including deet) that are sold in the United States (6).
Picaridin's activity is similar in effect to long-acting Extended U.S. Army Extended Duration Topical Insect and Arthropod Repellent (EDTIAR), which contains diethyltoluamide (deet) 35% (1). EDTIAR is sold in United States as Ultrathon (3M), and deet in its various formulations is the standard insect repellent used in the United States. Picaridin appears to be less irritating than deet (2); deet has limitations because its high potential to irritate eyes and mucous membranes makes application to the face difficult.
One field trial study compared three repellents including picaridin and deet, at night: 9.3% picaridin, 19.2% picaridin, and 35% deet in a gel (Australian Defense Force [ADF]); and during the day: 19.2% picaridin, 20% deet in a controlled release formulation (Sawyer Controlled Release deet), and EDTIAR, against rainforest mosquitoes in northern Queensland. Australia. In nighttime tests. 19.2% picaridin provided >94.7% protection for at least 9 hours, and ADF deet provided >95% protection for 7 hours. The 9.3% picaridin formulation provided >95% protection for only 2 hours, and provided 60% protection at 9 hours. In daytime tests. Sawyer 20% deet provided >95% protection for 6 hours, and both 19.2% picaridin and EDTIAR provided >95% protection for 8 hours. In both nighttime and daytime tests, 19.2% picaridin provided similar to deet (7).
Another Australian study found that picaridin is better tolerated than deet. In early 2001. 150 soldiers deployed to East Timor were asked to compare the ADF 35% deet gel formulation with 19.2% picaridin applied as a non-pressurized pump action spray. The soldiers were asked to use each formulation for one week, applying it twice a day. At the end of two weeks, they were asked to complete a questionnaire. Significantly more soldiers reported mild discomfort and irritation with the use of ADF deet compared with 19.2% picaridin (8).
In conclusion, picaridin is a promising new insecticide. Its effect is similar to deet, yet it appears to be less irritating than deet. As such it will be a valuable addition to the medical armamentarium in the prevention of insect bites.
References
1. Insect Repellents. The Medical Letter on Drugs and Therapeutics 2003 May 6; 45:41-42.
2. www.autan.com/objekte/bayrepel_brosch.pdf (June 7. 2003).
3. www.epa.gov/oppfead1/annual/2001/2001 annual.htm (June 7. 2003).
4. www.ces.uga.edu/Agriculture/entomology/pestnewsletter/NL-jan02.htm (June 7, 2003).
5. http://pest.ifas.ufl.edu/CMSP-2002/01cmsp02.htm (June 7, 2003).
6. www.ks-agr.org/pesticide/mosquito/personaluse.asp.
7. Frances SP. Van Dung N, Beebe NW, Debboun M. Field evaluation of repellent formulations against daytime and nighttime biting mosquitoes in a tropical rainforest in northern Australia. J Med Entomol 2002; 39:541-4.
8. Frances SP, Cooper RD. Personal protection measures against mosquitoes: A brief history and current use of repellents by the Australian Defence Forces. ADF Health September 2002; 3(2):58-63.
NOAH SCHEINFELD MD
ST. LUKE'S ROOSEVELT HOSPITAL CENTER AND BETH ISRAEL MEDICAL CENTER NEW YORK, NEW YORK
ADDRESS FOR CORRESPONDENCE:
Noah Scheinfeld MD
St. Luke's Roosevelt Hospital Center
Department of Dermatology
1090 Amsterdam Ave Suite 11D New York, NY 10025
Phone: (212) 523-3888
Fax: (212) 523-3808
E-mail: Scheinfeld@earthlink.net
COPYRIGHT 2004 Journal of Drugs in Dermatology
COPYRIGHT 2004 Gale Group