METHOD OF PREPARATION
Note: This preparation should be prepared in a laminar flow hood in a cleanroom (or via isolation barrier technology) by a validated aseptic compounding pharmacist using strict aseptic technique.
1. Calculate the required quantity of each ingredient for the total amount to be prepared.
2. Accurately weigh and/or measure each ingredient.
3. Mix the benzoyl benzoate and the dimercaprol.
4. Add sufficient peanut oil to final volume and stir until dissolved.
5. Filter through an appropriate sterile 0.2-gm filter into sterile vials or dry heat sterilize.
6. Package and label.
PACKAGING
Package in sterile vials.
LABELING
For professional use.
STABILITY
A beyond-use date of up to 6 months can be used for this preparation.1
USE
Dimercaprol injection is the drug of choice for an antidote to arsenic, gold, and mercury poisoning as a result of the ingestion of the salts of these metals. It is also used for the treatment of Lewisite or mustard-derivative mixture poisoning in the context of chemical warfare or terrorism.2
QUALITY CONTROL
Quality-control assessment can include weight and/or volume, physical observation, specific gravity, assay, color, clarity, particulate matter, sterility, and pyrogenicity testing.3,4
DISCUSSION
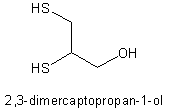
Dimercaprol (C^sub 3^H^sub 8^OS^sub 2^, MW 124.23) occurs as a colorless or practically colorless liquid with a disagreeable, mercaptan-like odor. It is soluble in water, alcohol, and benzyl benzoate. It has a specific gravity betwen 1.242 and 1.244 and a refractive index between 1.567 and 1.573.1
Dimercaprol Injection USP is a sterile solution of dimercaprol in a blend of benzyl benzoate and vegetable oil. It contains not less than 9.0 g and not more than 11.0 g of dimercaprol in each 100 g of mixture. The preparation is a yellow, viscous solution having a pungent, disagreeable odor and a specific gravity of about 0.978.1
Benzoyl benzoate (C^sub 14^H^sub 12^O^sub 2^, MW 212.24, benzyl phenylformate) is a solubilizing agent, solvent and plasticizer. It is also used in the treatment of scabies and is used as a parasiticide in veterinary medicine. It occurs as a clear, colorless, oily liquid with a slightly aromatic odor. It will crystallize at temperatures below 17 deg C. It has a specific gravity of 1.12, and a melting point of 21 deg C. It is practically insoluble in glycerin and water, but is miscible with 95 % ethanol and oils. It is rapidly metabolized to benzoic acid and benzyl alcohol in the body. It is incompatible with alkalis and oxidizing agents.5
Peanut oil (earthnut oil, groundnut oil, katchung oil, nut oil) is a colorless or pale yellow-colored liquid with a bland, nutty taste and a faint, nutty odor. It is generally composed of the glycerides of about 56% oleic acid, 26% linoleic acid, 8.3% palmitic acid, and less than 5% of arachidic acid, behenic acid, stearic acid, and lignoceric acid. It is used as an oleaginous vehicle and solvent. Specifically, it has been used as a prolonged-- release solvent for intramuscular injections, as a vehicle for topical preparations, and as a solvent for vitamins and hormones. It has even been used as a fecal softener in enemas and in ear drops to soften ear wax. It has a density of about 0.910 to 0.915 and a freezing point of -5 deg C. If cooled to about 3 deg C, it will become cloudy and partially solidify as the temperature is further lowered. It is very slightly soluble in 95% ethanol, soluble in oils, and miscible with chloroform. Peanut oil is relatively stable but can slowly thicken and become rancid upon exposure to air. It can be sterilized by filtration or dry heat and, if intended for use in parenteral dosage forms, should be stored in a glass container. Storage in well-filled, airtight, light-resistant containers is recommended. Therapeutically, it can be used as a laxative (15 mL to 60 mL orally, 100 mL to 500 mL rectally as an enema).6
References
1. US Pharmacopeial Convention, Inc. United States Pharmacopeia 25/National Formulary 20. Rockville, MD:US Pharmacopeial Convention, Inc; 2001:587-588, 2053-2057, 2375.
2. McEvoy GK. AHFS Drug Information-2002. Bethesda, MD:American Society of Health-Systems Pharmacists; 2002:2895-2897.
3. Allen LV Jr. Standard operating procedure for particulate testing for sterile products. IJPC 1998;2:78.
4. Allen LV Jr. Standard operating procedure: Quality assessment for injectable solutions. OPC 1999;3:406-407.
5. Daskalakis SA. Benzyl benzoate. In: Kibbe A, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC:American Pharmaceutical Association; 2000:44-45.
6. Kibbe AH. Peanut oil. In: Kibbe A, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC:American Pharmaceutical Association; 2000:360-361.
Copyright International Journal of Pharmaceutical Compounding Mar/Apr 2003
Provided by ProQuest Information and Learning Company. All rights Reserved