In nearly all cases of poisoning in which the health of a pregnant woman is in jeopardy and an antidote is needed, the woman's condition takes priority over the safety of the pregnancy and she should be treated the same as would a nonpregnant woman.
Only two antidotes--ethanol and penicillamine--pose a known risk to the embryo or fetus. But for most antidotes, the reported human pregnancy experience is typically limited to a few cases. For some, there is no human experience. The antidotes (targeted poisons shown in parentheses) are:
Naloxone, naltrexone, and nalmefene--a long-acting derivative of naltrexone with a plasma half-life of about 10 hours--are used to reverse narcotic overdose that is causing respiratory depression and/or marked sedation. All can be used in pregnancy.
Acetylcysteine (acetaminophen), used to prevent or lessen hepatic injury, is not teratogenic or embryotoxic in animals. Limited human pregnancy data suggest it is not toxic to the fetus. When administered intravenously, sufficient amounts cross the placenta to achieve protective serum levels in the fetus.
There are only two reports of IV digoxin immune Fab, ovine (digoxin) use in human pregnancy. Neither case involved a digoxin overdose.
Flumazenil (benzodiazepines) is not teratogenic or toxic to the embryo/fetus in animals at systemic exposures near those obtained in humans. It appears to cross the human placenta, and reverses the depressive effects of benzodiazepines in the fetus.
Fomepizole (ethylene glycol; methanol) was not teratogenic in mice studies, but only one case of human pregnancy exposure has been reported and the outcome was unknown. Shortterm use of ethanol also has been reported for poisonings with these two chemicals, but this use has not been studied in pregnancy. Neurotoxicity is a potential fetal complication.
There are five agents to treat heavy metal intoxication:
* Deferoxamine (acute and chronic iron) causes toxicity in two animal species, but human pregnancy experience is substantial, and no embryonic or fetal adverse effects attributable to the agent have been reported.
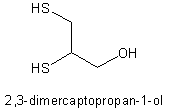
* Dimercaprol is either used alone (arsenic, gold, and acute mercury) or combined with edetate calcium disodium (lead). High doses are embryotoxic and teratogenic in mice. The published human pregnancy experience is limited to two arsenic and one lead poisoning cases, which both occurred after the first trimester. High levels of arsenic or lead were found in the newborns in two cases.
* Edetate calcium disodium (lead) is used either alone or in combination with dimercaprol. There are three reports of its use in human pregnancy, all late in gestation. A potential complication is maternal hypotension.
* Penicillamine is a chelating agent for copper in the treatment of Wilson's disease: there is one report of its use in mercury poisoning. First-trimester exposure is related to connective tissue anomalies (cutis laxa).
* Succimer (DSMA) (lead, arsenic, mercury, and cadmium) also chelates zinc. It is toxic and/or teratogenic in mice and rats, but some effects may have been due to zinc deficiency. Because of the absence of human pregnancy experience, other antidotes are preferable.
The reported human pregnancy experience with physostigmine (CNS effects of scopolamine, other anticholinergics, and tricyclic antidepressants) is limited to the last trimester. No adverse effects from the drug were seen.
Methylene blue (cyanide) is teratogenic and fetal toxic when given by intraamniotic injection in humans: its oral use as an antidote in pregnancy has not been reported. The effects of amyl nitrite, sodium nitrite, and sodium thiosulfate (the contents of the Cyanide Antidote Package) on human pregnancy also are unknown, as are the effects of high-dose hydroxocobalamin (cyanide).
Animal reproduction studies have not been conducted with commercially available antivenins for acute envenomations, and human reports are rare or completely absent.
Other antidotes include atropine (organophosphates and carbamates), calcium chloride or gluconate (calcium-channel-blocker, hydrofluoric acid), protamine (heparin), vitamin K (warfarin), folinic acid (methotrexate), and glucagon (hypoglycemia). The data for these agents do not suggest significant risk to the embryo or fetus.
Activated charcoal prevents absorption of substances from the gut and, if vomiting is prevented, presents no risk to the mother or her pregnancy. Similarly, syrup of ipecac, used to induce vomiting when indicated, is safe in pregnancy.
BY. GERALD G. BRIGGS, B. PHARM.
GERALD G. BRIGGS is pharmacist clinical specialist, Women's Hospital, Long Beach Memorial Medical Center; clinical professor of pharmacy, University of California, San Francisco; and adjunct professor of pharmacy, University of Southern California, Les Angeles. He is also coauthor of the reference book "Drugs in Pregnancy and Lactation."
COPYRIGHT 2004 International Medical News Group
COPYRIGHT 2004 Gale Group