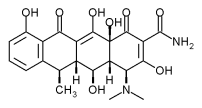
Skidmore R, Kovach R, Walker C, et al. Effects of subantimicrobial-dose doxycycline in the treatment of moderate acne. Arch Dermatol 2003; 139:459-464.
* PRACTICE RECOMMENDATIONS
The authors propose that moderate acne may be treated with doxycycline in subantimicrobial doses (20-mg tablets taken twice daily). This regimen was well-tolerated, moderately effective in reducing skin lesions, and did not have a detectable effect on the antibiotic resistance of skin flora.
The cost of Periostat (the only form of doxycycline 20 mg available in the US) is about $55 per month, while generic doxycycline 100 mg is about $10. (1) This study has some significant flaws, but a trial of low-dose doxycycline in an adult with acne severe enough to warrant antibiotics would still seem a reasonable, albeit expensive, option.
* BACKGROUND
Doxycycline, when used in doses that are below its antimicrobial threshold, is effective in treating adult chronic periodontitis. Doxycycline hyclate 20 mg twice daily (Periostat) is believed to work by decreasing inflammation and does not appear to create bacterial resistance to itself. Given the role of host inflammatory response in ache, the researchers tested sub-antimicrobial dosing of doxycycline in adults with moderate ache.
* POPULATION STUDIED
The subjects were 51 adults with moderate acne (defined as 6-200 comedones, 10-75 papules, and <6 nodules) drawn from 2 university-based outpatient clinics. Thirty-five (69%) were white, and the mean age was 23 years.
Subjects could not have used acne therapy for 6 weeks prior to enrollment and could not be on hormonal contraception. Forty of these patients completed the 6-month study. Of the patients not completing the study, 5 were lost to follow-up, 4 did not follow the study protocol, and 2 had possible adverse reactions to the study drug.
* STUDY DESIGN AND VALIDITY
In this double-blind randomized study, patients were given either oral doxycycllne hyclate 20 mg twice daily (n=26) or a matching placebo (n=25). Patients were assessed at baseline and at 2, 4, and 6 months.
The authors do not describe how the study participants were recruited into the study, and no information is given regarding allocation concealment. After randomization, the groups were not well-balanced, in that there were approximately twice as many men in the placebo group than the treatment group. Since androgens play a significant role in acne, this unequal distribution of men into the placebo group may partly be responsible for the better results found in the treatment group. It raises further doubt as to whether allocation assignment was concealed from the enrolling researchers.
Analyses appeared to be per protocol and not by intention-to-treat. The study was supported by the makers of Periostat.
* OUTCOMES MEASURED
The primary outcomes measured were the number of lesions present at each evaluation. Secondary outcomes included the clinician global assessment and the patient self-assessment scores using a 7-point qualitative scale. Forehead skin flora samples were collected at baseline and at 6 months to assess for changes in microflora or resistance patterns.
* RESULTS
Six months after baseline, the doxycycline group showed a greater decrease in inflammatory lesions (50.4% fewer vs 30.2% fewer for placebo; P=.04) and comedones (53.6% fewer vs 10.6% fewer; P=.01). The clinician's global assessment, however, was an improvement of only 0.4 on a 7-point scale at 6 months, a barely clinically significant difference (P=0.03). The change in patients' global assessment was also towards improvement, but was not statistically significant.
Two patients in the doxycycline group dropped out due to possible adverse drug reaction. One patient had a gastric ulcer with bleeding, which was not attributed to the medication. Another had a recurrence of yeast vaginitis, which was thought to be related to the antibiotic. No significant change was detected in the composition of the skin flora or in antibiotic susceptibility in those patients taking antibiotics.
REFERENCE
(1.) Drug prices from www.drugstore.com. Accessed on June 23, 2003.
Seth T. Miller, MD, and James J. Stevermer, MD, MSPH, Department of Family and Community Medicine, University of Missouri-Columbia. E-mail: Millerse@health.missouri.edu.
COPYRIGHT 2003 Dowden Health Media, Inc.
COPYRIGHT 2003 Gale Group