This is the second installment of a three-part series of columns on the safety of antibiotics in pregnant and lactating women.
* Lincosamides. Clindamycin (Cleocin) has been classified under pregnancy category B. There is no evidence of teratogenicity or embryo-ferotoxicity from exposure during any stage of gestation. This drug is commonly used during pregnancy and is safe to use during breast-feeding.
* Quinolones and fluoroquinolones. Of the older quinolones, nalidixic acid (NeGram) is rated category C. Nalidixic acid is still used to treat urinary tract infections but not widely It is teratogenic and embryocidal in rats when given at about six times the human dose. It should therefore be avoided in the first trimester if possible, but there is no evidence of fetal harm in humans. Cinoxacin (Cinobac) is rated category B, but there are no fetal risk or breast-feeding data on this drug.
All 10 of the currently available fluoroquinolones are rated C. These include ciprofloxacin (Cipro), enoxacin (Penetrex), gatifloxacin (Tequin), 1evofloxacin (Levaquin), lomefloxacin (Maxaquin), moxifloxacin (Avelox), norfloxacin (Noroxin), ofloxacin (Floxin), and sparfioxacin (Zagam). They are not teratogenic or embryofetotoxic in rats, but they have been shown to have toxic effects (abortions and stunted growth) in rabbits exposed in utero to doses at or close to maternal toxic doses. But in observational studies in pregnant women, no association between fluoroquinolones and birth defects has been found.
All quinolones and fluoroquinolones can induce arthropathies in immature animals when given directly, and there have been case reports of lesions in cartilage in human children who have taken fluoroquinolones.
Based on the limited data, the safest course is to avoid these drugs during pregnancy. There usually are safer alternatives available, and they are not a primary drug for any infection commonly seen in pregnancy (with the exception of anthrax). If exposure occurs, however, pregnancy termination is not warranted.
For nursing women, there is the theoretical risk of arthropathies in babies exposed to the drugs in breast milk and the known risk of phototoxicity. Phototoxicity has been reported in adults on these drugs, and squamous cell carcinoma has been reported in infant mice exposed chronically to some fluoroquinolones and periodically to ultraviolet light. Therefore, I recommend against breast-feeding when taking fluoroquinolones and advise waiting for 48 hours after taking the last dose to breast-feed.
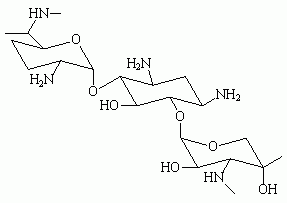
* Aminoglycosides. The seven drugs in this class, which include amikacin (Amikin), kanamycin (Kantrex), strepromycin, tobramycin (Nebcin), neomycin, and gentamicin (Garamycin), are rated C or D. All cross the placenta. They are not teratogenic in humans but can produce dose-related ototoxicity and renal toxicity in adults. Kanamycin and streptomycin have been associated with hearing loss in babies exposed to the drug in utero. High, prolonged maternal doses led to the fetal toxicity.
Ototoxicity and renal toxicity may potentially occur with other aminoglycosides. Maternal serum levels of aminoglycosides should be closely monitored to ensure appropriate peak and trough levels. Combining an aminoglycoside with other ototoxic or nephrotoxic drugs should be avoided. I am not aware of any reports of ototoxicity or renal toxicity when maternal levels are in the appropriate range. It is advisable to prescribe gentamicin in a divided dose when given near term or during labor, because a single dose daily used close to delivery results in high and persistent levels in the newborn.
Another concern is an interaction that may occur between aminoglycosides and magnesium sulfate. When a mother is treated with magnesium sulfate just before delivery, and then the newborn is treated with an aminoglycoside a few hours after birth, neuromuscular weakness and paralysis may occur in the infant.
All aminoglycosides are compatible with breast-feeding.
* Vancomycin. Vancomycin appears to be safe during human pregnancy, but reports are limited. Fetal ototoxicity and nephrotoxicity are theoretical concerns with prolonged, high doses. If combined with an aminoglycoside, ototoxicity is possible. This has not been reported in infants, so monitoring serum levels is important. Vancomycin is compatible with breastfeeding.
GERALD G. BRIGGS, B.PHARM., is pharmacist clinical specialist, Women's Hospital, Long Beach Memorial Medical Center; clinical professor of pharmacy, University of California, San Francisco; and adjunct associate professor of pharmacy, University of Southern California, Los Angeles. He is also coauthor of the textbook "Drugs in Pregnancy and Lactation."
COPYRIGHT 2001 International Medical News Group
COPYRIGHT 2002 Gale Group