Business Editors/Health/Medical Writers
VANCOUVER, Canada--(BUSINESS WIRE)--Aug. 14, 2003
A meta-analysis of 13 trials, involving 4,556 patients with advanced non-small cell lung cancer, demonstrates statistically-significant improvement in overall survival and median progression-free survival for Gemzar(R)/platinum-based therapies when compared with other platinum-based regimens. The results of this analysis were presented today at the 10th World Congress on Lung Cancer (WCLC) in Vancouver, Canada.
Meta-analyses statistically combine the results of several studies into single outcome measures. This approach increases the overall sample size which, in turn, increases the statistical power of the analysis. However, conclusions are limited to the published data and patient populations included in the meta-analysis.
Platinum compounds, such as cisplatin and carboplatin, are referred to as first-generation cancer therapies and are frequently combined with newer agents in an effort to improve patient outcomes.
According to researchers who conducted this meta-analysis, an overall survival improvement was shown in favor of the Gemzar/platinum arms (hazard ratio 0.90) (p less than 0.001). This translates into a survival benefit after one year of 38.9 percent versus 35 percent. The data also demonstrated that Gemzar/platinum treated patients had a median progression-free survival of 5.1 months versus 4.4 months for the patients treated with the other chemotherapy regimens. Both of these observations were statistically significant.
Many respected oncologists and leading statisticians were part of this global research project supported by Eli Lilly and Company, with the actual analysis conducted by a third-party clinical research organization. The results were presented today by one of the authors, Dr. Joan H. Schiller, professor of medicine, University of Wisconsin, and chair of the lung cancer committee of the Eastern Cooperative Oncology Group, an NCI-funded network of cancer researchers at cancer care institutions across the US.
"This meta-analysis confirms the role Gemzar plays as a standard-of-care agent worldwide in the treatment of non-small cell lung cancer," said co-author, Dr. Christian Manegold, professor of medicine, University of Heidelberg, Germany.
"These data confirm the efficacy of Gemzar, which has been tested in more Phase III randomized studies than paclitaxel, docetaxel or vinorelbine. As lung cancer remains the number one cancer killer in the world, these results provide important observations that will assist oncologists in practicing evidence-based medicine," said Manegold.
A comprehensive review of published clinical study reports identified 13 randomized trials of Gemzar plus cisplatin or carboplatin versus other platinum-based combinations. Accordingly, a meta-analysis of overall survival and progression-free survival was performed. Seventeen platinum-based comparators from 13 trials were included in the meta-analysis.
"This study is a significant addition to the formidable body of evidence demonstrating the clinical performance of Gemzar," said Paolo Paoletti, M.D., vice president of oncology products at Eli Lilly and Company. "We believe these data validate the utility of Gemzar in first-line non-small cell lung cancer treatment and confirm our belief that doctors can achieve desired overall outcomes for their patients by using Gemzar-based therapy."
Gemzar
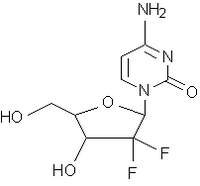
Gemzar(1) is approved in more than 90 countries. It is the standard of care worldwide for pancreatic cancer. Gemzar is also the standard of care in many parts of the world for non-small cell lung and bladder cancers. Gemzar is currently approved in combination with cisplatin in NSCLC in most countries where it is available, and in many countries, it is also approved as a single agent in NSCLC. Also approved in some European countries for breast cancer, Gemzar is a nucleoside analogue that interferes with the processes of DNA production; by doing so, Gemzar prevents cancer cells from replicating and thus slows or stops tumor growth.
Lilly, a leading innovation-driven corporation is developing a growing portfolio of best-in-class pharmaceutical products by applying the latest research from its own worldwide laboratories and from collaborations with eminent scientific organizations. Headquartered in Indianapolis, Ind., Lilly provides answers - through medicines and information - for some of the world's most urgent medical needs.
Gemzar(R) (gemcitabine hydrochloride, Lilly)
Navelbine(R) (vinorelbine, Glaxo Wellcome)
Taxol(R) (paclitaxel, Bristol-Myers Squibb Company)
Taxotere(R) (docetaxel, Aventis Pharmaceuticals, Inc.)
(1) For an appropriate use of Gemzar, please refer to the Summary
of Product Characteristics
COPYRIGHT 2003 Business Wire
COPYRIGHT 2003 Gale Group