Business Editors/Health/Medical Writers
INDIANAPOLIS--(BUSINESS WIRE)--June 23, 2003
Eli Lilly and Company announced today that regulatory officials in Finland approved an expanded indication for Gemzar(R) (gemcitabine HCl) in combination with Taxol(R) (paclitaxel) for the treatment of patients with unresectable, locally recurrent or metastatic breast cancer who have relapsed following adjuvant or neoadjuvant chemotherapy. Based on a submission in December, this indication requires prior chemotherapy and should have included an anthracycline unless clinically contraindicated.
Breast cancer is diagnosed in more than 200,000 women in the European Union each year and is the third most common malignancy worldwide(1).
"Gemzar has proven its efficacy in clinical trials that support multiple indications in solid tumour treatment. This combination offers women an increased amount of time until the disease spreads or worsens," said Paolo Paoletti, M.D., vice president of oncology products, Eli Lilly and Company. "As we watch the incidence of breast cancer grow each year it is increasingly important to continue to discover new combinations to combat this disease."
Approval is based on interim analysis of data gathered from an ongoing Phase III trial of the Gemzar/Taxol combination compared to single-agent Taxol in patients with metastatic breast cancer. A U.S. regulatory submission for Gemzar in the use of metastatic breast cancer is currently being discussed. The primary endpoint of this trial is overall survival.
These interim results were recently presented at the 39th annual meeting of the American Society of Clinical Oncology in Chicago earlier this month. The global study of 529 women who were previously treated with an anthracycline with no prior chemotherapy in the metastatic setting yielded the following results:
-- Gemzar/Taxol combination significantly delayed progression of
the disease compared to single-agent Taxol (5.4 months vs. 3.5
months, p= 0.0013). Time to disease progression is a measure
of time after cancer is treated until the disease starts to
get worse.
-- Overall response rate, which measures the percentage of
patients whose cancer shrinks or disappears after treatment,
was also significantly better with the Gemzar/Taxol
combination (39.3 percent vs. 25.6 percent, p= 0.0007).
-- Non-hematologic toxicity was moderate in both arms. Grade IV
hematologic toxicity was more pronounced in the combination
arm, with the most common side effect being a decrease in
white blood cells (17.2 percent vs. 6.6 percent, p= 0.0002)
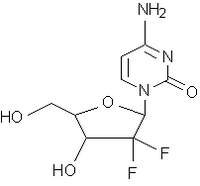
Gemzar is approved in more than 90 countries. It is the standard of care worldwide for pancreatic cancer. Gemzar is also a standard of care in many parts of the world for non-small-cell lung and bladder cancers. Gemzar is a nucleoside analogue that interferes with the processes of DNA production; by doing so, Gemzar prevents cancer cells from replicating and thus slows or stops tumour growth.
Lilly, a leading innovation-driven corporation, is developing a growing portfolio of best-in-class pharmaceutical products by applying the latest research from its own worldwide laboratories and from collaborations with eminent scientific organizations. Headquartered in Indianapolis, Ind., Lilly provides answers - through medicines and information - for some of the world's most urgent medical needs.
Gemzar(R) (gemcitabine hydrochloride, Lilly)
Taxol(R) (paclitaxel, Bristol-Myers Squibb Oncology/Immunology)
(1) Europa Donna - The European Breast Cancer Coalition
COPYRIGHT 2003 Business Wire
COPYRIGHT 2003 Gale Group