METHOD OF PREPARATION
1. Calculate the required quantity of each ingredient for the total amount to be prepared.
2. Accurately weigh and/or measure each ingredient.
3. Pulverize the required quantity of misoprostol tablets to a very fine powder.
4. Incorporate the gentamicin sulfate and phenytoin powders and mix well.
5. Add the parabens to the propylene glycol, followed by the blended powders.
6. Incorporate the hydroxyethylcellulose powder and mix well.
7. Add the mixture to about 80 mL of purified water and mix well. If necessary, heat the water to about 70°C and mix well.
8. Add sufficient purified water to volume and mix well.
9. Package and label.
PACKAGING
Package in tight, light-resistant containers.1
LABELING
Keep out of reach of children. Use only as directed. For external use only.
STABILITY
A beyond-use date of 14 days, when stored in a refrigerator, can be used for this preparation.1
USE
This preparation is used in the topical treatment of decubitus ulcers and other open wounds.
QUALITY CONTROL
Quality-control assessment can include weight/volume, pH, specific gravity, active drug assay, color, clarity, rheological properties, physical observation and physical stability (discoloration, foreign materials, gas formation, mold growth).2
DISCUSSION
Preparations containing phenytoin and misoprostol in combination with various antibiotics are now in general use in the treatment of open wounds, especially decubitus ulcers. The selection of the antibiotic for these combinations is dependent upon the susceptible microorganisms involved.
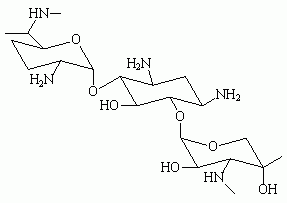
Gentamicin sulfate is an aminoglycoside antibiotic occurring as the sulfate salt, or a mixture of such salts, of the antibiotic substances produced by the growth of Micromonospora purpurea. Commercially, it is a mixture of the sulfate salts of gentamicin C^sub 1^, C^sub 2^ and C^sub 1A^. It occurs as a white-to-buff-colored powder that is soluble in water but insoluble in alcohol. It has a potency equivalent of not less than 590 µg of gentamicin per mg calculated on the dried basis.1,3
Misoprostol (C^sub 22^H^sub 38^O^sub 5^, MW 382.53, Cytotec) is a synthetic analog of prostaglandin E^sub 1^ (alprostadil) and occurs as a water-soluble viscous liquid. It is a gastric antisecretory agent with protective effects on the gastroduodenal mucosa. Cytotec tablets contain either 100 µg or 200 µg of misoprostol. The tablets also contain hydrogenated castor oil, hydroxypropyl methylcellulose, macrocrystalline cellulose and sodium starch glycolate.4
Phenytoin (C^sub 15^H^sub 12^N^sub 2^O^sub 2^, MW 252.27, 5,5-diphenylhydantoin) occurs as a white, odorless powder. It is practically insoluble in water. It should be preserved in tight containers.1,3
Hydroxyethylcellulose (HEC) occurs as a light tan or cream-to-white-colored, odorless and tasteless powder. Solutions can be easily made by dispersing HEC in mildly agitated water at room temperature. When the powder has been thoroughly wetted, increasing the temperature to 60-70°C speeds up the dispersion process.5
Methylparaben (C^sub 8^H^sub 8^O^sub 3^, MW 152.15, methyl hydroxybenzoate, methyl parahydroxybenzoate,) is available as colorless crystals or as a white, crystalline powder that is odorless, or almost odorless, and has a slight burning taste.6
Propylparaben (C^sub 10^H^sub 12^O^sub 3^, MW 18.20, propyl hydroxybenzoate, propyl parahydroxybenzoate,) is available as a white, crystalline, odorless and tasteless powder.6
Propylene glycol (C^sub 3^H^sub 8^O^sub 2^, MW 76.09) occurs as a clear, colorless, viscous, practically odorless liquid with a sweet taste, somewhat resembling glycerin.7
Purified water is water that is obtained by distillation, ion exchange, reverse osmosis or some other suitable process.8
REFERENCES
1. US Pharmacopeial Convention, Inc. United States Pharmacopeia 27-National Formulary 22. Rockville, MD: US Pharmacopeial Convention, Inc.; 2004: 859-860, 2345-2349, 2776.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of oral and topical liquids. IJPC 1999; 3: 146-147.
3. McEvoy GK. AHFS Drug Information-2000. Bethesda, MD: American Society of Health-System Pharmacists; 2000: 70-72, 1972-1975.
4. [No author listed.] Physicians' Desk Reference. 56 ed. Montvale, NJ: Medical Economics Company; 2002: 3202-3203.
5. Harwood RJ. Hydroxyethyl cellulose. In: Kibbe AH, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC: American Pharmaceutical Association; 2000: 240-243.
6. Reiger MM. Methylparaben and Propylparaben. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC: American Pharmaceutical Association; 2003: 390-394, 450-453.
7. Weller PJ. Propylene glycol. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC: American Pharmaceutical Association; 2003: 521-523.
8. Ellison A, Nash RA, Wilkin MJ. Water. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC: American Pharmaceutical Association; 2003: 672-676.
Copyright International Journal of Pharmaceutical Compounding Nov/Dec 2004
Provided by ProQuest Information and Learning Company. All rights Reserved