Gleevec
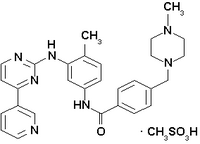
Imatinib is a drug used to treat certain types of cancer. It is currently marketed by Novartis as Gleevec® (USA) or Glivec® (Europe/Australia) as its mesylate salt, imatinib mesilate (INN). It is occasionally still referred to as CGP57148B or STI571 (especially in older publications). It is used in treating chronic myelogenous leukemia (CML), gastrointestinal stromal tumors (GISTs) and a number of other malignancies. more...
It is the first member of a new class of agents that act by inhibiting particular tyrosine kinase enzymes, instead of simply inhibiting rapidly dividing cells.
Molecular biology
Imatinib is a 2-phenylaminopyrimidine derivative that functions as a specific inhibitor of a number of tyrosine kinase enzymes. It occupies the TK domain, leading to a decrease in activity.
There are a large number of TK enzymes in the body, including the insulin receptor. Imatinib is specific for the TK domain in abl (the Abelson proto-oncogene), c-kit and PDGF-R (platelet-derived growth factor receptor).
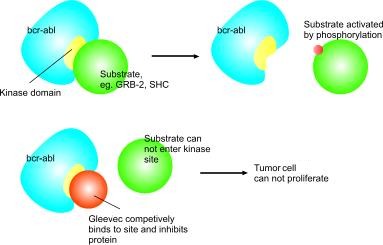
In chronic myelogenous leukemia, the Philadelphia chromosome leads to a fusion protein of abl with bcr (breakpoint cluster region), termed bcr-abl. As this is now a continuously active tyrosine kinase, Imatinib is used to decrease bcr-abl activity.
Imatinib works because p210bcr-abl requires a molecule of ATP to activate tyrosine residues on its substrates by phosphorylation. Imatinib instead docks in to this site and inhibits the protein competitively. Imatinib is quite selective for bcr-abl – it does also inhibit other targets mentioned above, but no known other tyrosine kinases. Imatinib does of course work on the abl protein of all cells but these have additional, normally redundant, pathways which allow the cell to continue to function normally even without this one. Tumour cells, however, have a dependence on bcr-abl (Deininger and Druker, 2003). Inhibition of the bcr-abl tyrosine kinase also stimulates its entry in to the nucleus, where it is unable to perform any of its normal anti-apoptopic functions (Vigneri et al 2001).
Uses
Imatinib is used in chronic myelogenous leukemia (CML), gastrointestinal stromal tumors (GISTs) and a number of other malignancies. Early clinical trials also show its potential for treatment of hypereosinophilic syndrome and dermatofibrosarcoma protuberans.
In laboratory settings, imatinib is being used increasingly as an experimental agent to suppress platelet-derived growth factor (PDGF) by inhibiting its receptor (PDGF-Rβ). One of its effects is delaying atherosclerosis in mice with diabetes (Lassila 2004).
Recent mouse animal studies at Emory University in Atlanta have suggested that imatinib and related drugs may be useful in treating smallpox, should an outbreak ever occur.
Tolerance
In the United States, the Food and Drug Administration has approved imatinib as first-line treatment for CML (Deininger and Druker 2003). Imatinib has passed through Phase III trials for CML, and has been shown to be more effective than the previous standard treatment of α-interferon and cytarabine. Although the long-term side effects of imatinib have not yet been ascertained, research suggests that it is generally very well tolerated (eg. liver toxicity was much less than predicted). Broadly, side effects such as edema, nausea, rash and musculoskeletal pain are common but mild.
Read more at Wikipedia.org