Business Editors
BALTIMORE, Md.--(BUSINESS WIRE)--Sept. 19, 2002
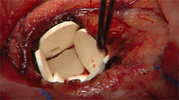
Guilford Pharmaceuticals (NASDAQ: GLFD), will host a conference call and webcast to discuss long term survival results in a Phase III clinical trial of GLIADEL(R) Wafer, a proprietary treatment for brain cancer. The conference call will take place at 1:00 p.m. Eastern Time on Thursday, September 19, 2002. The dial in number for U.S. residents is (888) 425-2604, and international residents, (706) 679-8253.
This call is being webcast by CCBN and can be accessed at Guilford's web site at www.guilfordpharm.com, under investors/presentations.
The webcast is also being distributed over CCBN's Investor Distribution Network to both institutional and individual investors. Individual investors can listen to the call through CCBN's individual investor center at www.companyboardroom.com or by visiting any of the investor sites in CCBN's Individual Investor Network. Institutional investors can access the call via CCBN's password-protected event management site, StreetEvents (www.streetevents.com).
About Guilford Pharmaceuticals
Guilford Pharmaceuticals Inc. is a fully integrated pharmaceutical company that discovers, develops and markets novel pharmaceutical products targeting the hospital and neurological markets.
COPYRIGHT 2002 Business Wire
COPYRIGHT 2002 Gale Group