Guilford Pharmaceuticals Inc. (NASDAQ: GLFD) announced today that its
supplemental New Drug Application (sNDA) to the U.S. Food and Drug
Administration (FDA) to seek approval to expand the labeled
indication for GLIADEL(R) Wafer (polifeprosan 20 with carmustine
implant) to include first line therapy in patients newly diagnosed
with malignant glioma has been accepted for review by the FDA. The
sNDA for GLIADEL(R) Wafer contains all required elements and the
agency has indicated it will be proceeding with a priority review.
The first new therapy to be approved for the treatment of brain cancer
in over two decades, GLIADEL(R) Wafer received marketing approval
from the U.S. Food and Drug Administration in September 1996 to treat
recurrent glioblastoma multiforme (GBM), a common form of malignant
brain cancer. Presently, GLIADEL(R) Wafer is approved for use for
recurrent surgery in 21 countries worldwide.
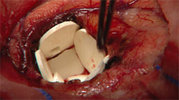
GLIADEL(R) Wafer is a small, white dime-sized wafer made of a
biodegradable polymer that contains the cancer chemotherapeutic drug,
carmustine or BCNU. Up to eight GLIADEL(R) Wafers can be implanted
in the cavity created when a brain tumor is surgically removed.
There, the wafers slowly dissolve over a period of 2 to 3 weeks,
delivering chemotherapy directly to the tumor site in high
concentrations, while minimizing drug exposure to other areas of the
body.
"Each year in the United States there are approximately 8,000 - 12,000
surgeries performed to treat malignant brain cancer. Unfortunately,
the recurrence rate for GBM is very high and approximately one third
of these patients will experience a recurrence of their primary tumor
and require a second surgery," commented Dr. Craig R. Smith,
President and Chief Executive Officer. "Presently, GLIADEL(R) Wafer
is approved to treat only the 30% of patients who experience tumor
recurrence. However, should GLIADEL(R) Wafer receive approval from
the FDA for use at the time of initial diagnosis and surgery, we're
hopeful that we can bring the treatment benefits of localized
chemotherapy with GLIADEL(R) Wafer to more patients worldwide."
GLIADEL(R) Wafers have generally been well tolerated. However,
GLIADEL(R) Wafer contains carmustine and should not be given to
patients who are allergic to carmustine. Carmustine can also cause
fetal harm when administered to a pregnant woman. Patients undergoing
surgery for malignant brain cancer and implantation of GLIADEL(R)
Wafer should be monitored closely for the following known
complications of surgery including: seizures, intracranial
infections, abnormal wound healing, and brain edema. Each of these
complications has been associated with GLIADEL(R) Wafer treatment.
Guilford Pharmaceuticals Inc. is a biopharmaceutical company engaged in
the development of biopolymer-based therapeutics for cancer and other
diseases, and novel products for the diagnosis and treatment of
neurological disorders.
For more information about GLIADEL(R) Wafer, visit www.gliadel.com.
Internet addresses: www.guilfordpharm.com
This press release contains forward-looking statements that involve
risks and uncertainties, including those described in the section
entitled "Risk Factors" contained in the Company's Annual Report on
Form 10-K for the year ended December 31, 2000, that could cause the
Company's actual results and experience to differ materially from
anticipated results and expectations expressed in these
forward-looking statements. Among other things, there can be no
assurance that the results of the third multi-center Phase III
clinical trial will support approval of GLIADEL(R) Wafer in the U.S.,
Europe and elsewhere for treatment of patients with malignant glioma
at the time of first surgery.