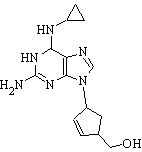
HIV-positive patients who have discontinued using the antiretroviral drug abacavir for any reason should not be restarted without first assessing the possibility of severe and fatal hypersensitivity reactions, Glaxo Wellcome Inc. (Research Triangle Park, N.C.) stated in a safety update.
Severe and fatal hypersensitivity reactions have occurred within hours of reintroducing the drug in patients with no identified history or unrecognized symptoms of hypersensitivity; 20% of patients with reactions had respiratory symptoms including cough, dyspnea, and pharyngitis, but the presentation of hypersensitivity varies widely and can include fever, rash, gastrointestinal symptoms, and malaise.
If previous hypersensitivity is suspected, abacavir should not be reintroduced. When clinical presentation of an acute illness cannot be clearly differentiated from a hypersensitivity reaction, Ziagen should be permanently discontinued. Package inserts have been revised accordingly.
COPYRIGHT 2000 International Medical News Group
COPYRIGHT 2001 Gale Group