Business/Health Editors
BRIDGEWATER, N.J.--(BUSINESS WIRE)--Nov. 22, 2002
Enzon, Inc. (Nasdaq:ENZN) today announced the completion of the Company's acquisition of the North American rights to Abelcet(R) (Amphotericin B Lipid Complex Injection) from Elan Corporation, plc (NYSE: ELN).
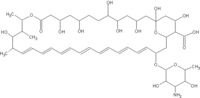
ABELCET is an antifungal used in the hospital to treat patients with invasive fungal infections related to cancer, organ transplantation and other conditions. Enzon has paid to Elan a cash payment of $360 million, representing the final purchase price.
The purchase includes the operating assets associated with the development, manufacture, sales and marketing of ABELCET in North America, including a 56,000 square foot manufacturing facility in Indianapolis, Indiana. Additionally, Enzon has hired certain Elan sales and plant personnel as part of the acquisition.
"This transaction does much more than simply add to Enzon's top and bottom lines, it brings a fully operational and profitable business to Enzon," said Arthur J. Higgins, Enzon's chairman and chief executive officer. "We have firmly anchored our strategy with the acquisition of ABELCET, an established product that plays a well-defined and valuable role in the growing antifungal market. Furthermore, this transaction sets the stage for continued growth bringing Enzon the necessary infrastructure and added flexibility to continue to access late-stage and marketed products."
The two companies have also agreed that for a maximum period of one year and at the option of Enzon, Elan will continue to market ABELCET in Canada and Elan will pay Enzon a royalty. The two companies have also entered into long-term Manufacturing and Supply Agreements, whereby Enzon will continue to manufacture products for Elan, including ABELCET, as Elan will retain the rights to market ABELCET in any markets outside the United States, Canada, and Japan. As part of the sale, Elan has committed to transfer its Japanese rights to Abelcet, to the extent they exist and are transferable to Enzon.
ABELCET is a lipid complex formulation of amphotericin B providing patients with significantly lower kidney toxicity than conventional amphotericin B. The increase in severe fungal infections is driven primarily by advances in medical treatment, such as increasingly aggressive chemotherapy procedures and advances in transplantation procedures. These advances have given rise to an increased number of immuno-compromised patients. The fungi that cause these infections are normally combated by an individual's healthy immune system. However, for immuno-compromised patients, such as individuals undergoing chemotherapy, immunosuppression following transplantation, or HIV patients with AIDS, these infections represent a major mortality risk.
SG Cowen Securities Corporation acted as financial advisor to Enzon.
Company management of Enzon will be hosting a conference call on Monday, November 25, 2002 at 10:00AM EST provide additional financial guidance related to the Company's acquisition of ABELCET. All interested parties can access the call using the following information:
Enzon's conference call will also be webcast in a "listen only" mode via the internet at http://www.vcall.com. Additionally, for those parties unable to listen at the time of Enzon's conference call, a rebroadcast will be available following the call from Monday November 25, 2002 from approximately 3:30PM EST. This rebroadcast will end on Monday, December 2, 2002 at 11:59PM EST. The rebroadcast may be accessed using the following information:
Enzon is a biopharmaceutical company dedicated to the discovery, development and commercialization of therapeutics to treat life-threatening diseases. The company has developed three marketed products, including PEG-INTRON(R), marketed by Schering-Plough. Enzon's product-focused strategy includes an extensive drug development program that leverages the Company's PEG modification and single-chain antibody (SCA(R)) technologies. Internal research and development efforts are complemented by strategic transactions that provide access to additional products, projects, and technologies. Enzon has several drug candidates in various stages of development, independently and with partners.
Except for the historical information herein, the matters discussed in this news release include forward-looking statements that may involve a number of risks and uncertainties. Actual results may vary significantly based upon a number of factors, which are described in the Company's Form 10-K, Form 10-Q's, Form 8-K's and other documents on file with the SEC, including without limitation, risks in obtaining and maintaining regulatory approval for existing, new or expanded indications and other regulatory risks, risks relating to the manufacturing facility and Enzon's ability to manufacture pharmaceutical products on a large scale, risks that customer inventory will be greater than previously thought, risks concerning Enzon's ability to manage growth, market a pharmaceutical product on a large scale and integrate and manage an internal sales and marketing organization and maintain or expand sales and market share for ABELCET, risks relating to the ability to ensure regulatory compliance, risks that Enzon will not outperform the sector, risks related to the acquisition, risks related to research and development costs and capabilities, market acceptance of and continuing demand for Enzon's products and the impact of increased competition, risks associated with anticipated top and bottom line growth and the possibility that upside potential will not be achieved, competitive products and pricing, and risks associated with the ownership and use of intellectual property rights.
This release is also available at http://www.enzon.com.
COPYRIGHT 2002 Business Wire
COPYRIGHT 2002 Gale Group