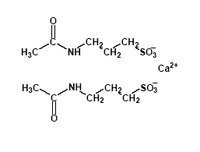
Approximately one-half of alcoholic patients relapse within three months of completing treatment. Several neurochemical agents may influence the mechanism of alcoholic cravings and relapse. Although the mechanisms are unknown, some studies have reported promising results with drugs that influence transmission of serotonin, dopamine and gamma-aminobutyric acid. Whilworth and colleagues conducted a double-blind, placebo-controlled study to assess the efficacy and safety of longterm use of acamprosate (calcium acetylhomotaurinate) in the treatment of alcohol dependency.
Patients treated by five Austrian hospitals for chronic or episodic alcoholism of at least 12 months' duration were eligible for the study. Alcoholism was validated by the CAGE questionnaire, the Michigan alcoholism screening test, and elevated liver function values and mean corpuscular volume. Patients with severe coexistent disease were excluded from the study. A total of 455 patients who had completed five days of alcohol withdrawal treatment were randomly assigned to receive either acamprosate or placebo. Patients were assessed on day 0 and on days 30, 90, 180, 270 and 360 for evidence of relapse and occurrence of side effects. The duration of treatment was 360 days. Patients were reassessed at 450, 540, 630 and 720 days. Seven patients recruited for the study did not receive medication and were therefore not included. Of the 448 remaining patients, 179 patients completed 12 months of treatment and 148 patients were followed for an additional year after the trial.
The placebo and treatment groups did not differ significantly in measures of severity of alcoholism and depression, or in demographic variables. By 360 days, 41 (18.3 percent) of the acamprosate-treated patients and 16 (7.1 percent) of placebo-treated patients had been continuously abstinent. The numbers and reasons for withdrawal from the trial were similar in both groups of patients. Relapse was the most common reason for withdrawal, and one-half of the relapses occurred within the first 90 days of treatment. The time to treatment failure continued to show a significant difference in favor of acamprosate. By the end of the two-year study, 27 patients (11.9 percent) in the acamprosate-treated group and 11 patients (4.9 percent) in the placebo-treated group remained continuously abstinent. No significant differences in side effects, with the exception of diarrhea, were detected between the two patient groups. Diarrhea was reported by 20.1 percent of patients taking acamprosate, compared with 12.1 percent of the placebo group.
The authors conclude that although treatment of alcoholism is notoriously difficult, acamprosate can be a safe and effective adjunct to rehabilitation programs. The authors believe that treatment should include a psychosocial element as well as a pharmacologic approach. The development of such treatments has the potential to benefit patients, reduce health care costs and alleviate the enormous social costs of alcoholism. (Whitworth AB, et al. Comparison of acamprosate and placebo in long-term treatment of alcohol dependence. Lancet 1996;347:1438-42.)
COPYRIGHT 1996 American Academy of Family Physicians
COPYRIGHT 2004 Gale Group