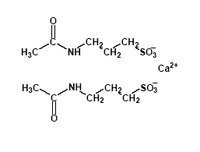
The drug Campral (acamprosate) has been approved by the FDA for treating alcohol-dependent individuals who want to continue to remain alcohol-free after they have stopped drinking. Campral is the first new drug approved for alcohol abuse in a decade.
Alcoholism, or alcohol dependence, is a disease. The consequences of alcohol misuse are serious and, in many cases, life threatening. Heavy drinking can increase the risk for certain cancers, especially those of the liver, esophagus, throat, and voice box (larynx). Heavy drinking can also cause liver cirrhosis, immune system problems, brain damage, and harm to the fetus during pregnancy. Chronic alcoholism continues to be a widespread and debilitating disorder that places a tremendous burden on society in terms of health care costs, lost wages, and personal suffering.
How Campral works is not fully understood, but the drug is thought to act on the brain pathways related to alcohol abuse. Campral was demonstrated to be safe and effective by multiple clinical studies involving alcohol-dependent people who had already been withdrawn from alcohol (detoxified). Campral proved superior to an inactive substance (placebo) in maintaining abstinence. This was indicated by a greater percentage of people who were treated with the drug being assessed as continuously keeping off alcohol consumption throughout treatment.
The most common side effects reported for patients taking Campral in clinical trials included headache, diarrhea, flatulence, and nausea. Campral is not addicting.
Campral may not be effective in people who are actively drinking at the start of treatment, or in people who abuse other substances in addition to alcohol. Treatment with Campral should be part of a comprehensive management program that includes psychosocial support.
Campral is manufactured by Merck KGaA of Darmstadt, Germany, and will be distributed in the United States by Forest Laboratories Inc. of New York City.
COPYRIGHT 2004 U.S. Government Printing Office
COPYRIGHT 2004 Gale Group