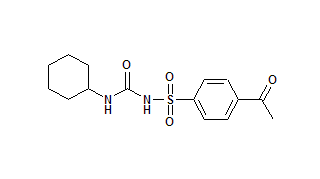
The Food and Drug Administration (FDA) has asked generic drug manufacturers to differentiate similar drug name pairs. For the past several years, the Institute for Safe Medication Practices (ISMP) has suggested using uppercase and lowercase letters (for example, chlorproMAZINE and chlorproPAMIDE) to distinguish drugs with look-alike names, especially when drugs share similar strengths.
The FDA sent letters to companies that manufacture acetazolamide, acetohexamide, chlorpropramide, clomipramine, daunorubicin, dobutamine, and dopamine, requesting that the companies use the uppercase and lowercase method.
Copyright Springhouse Corporation Mar 2002
Provided by ProQuest Information and Learning Company. All rights Reserved