ABSTRACT The effects of neuroactive compounds on larval metamorphosis of the Japanese short neck clam Ruditapes philippinarum (Heterodonta. Veneddaet were investigated by exposing pediveligers to acetylcholine, serotonin, epinephrine, norepinephrine, dopamine, L-3,4-dihydroxyphenylalanine (L-DOPA), carbamylcholine, and succinylcholine at concentrations of 1. 10, and 100 [micro]M. Larval metamorphosis with 100 [micro]M serotonin was 80.7%, and that with 10[micro]M and 100 [micro]M acetylcholine was 92.9% and 70.6%. respectively. No significant differences were observed in the induction activities of epinephrine (35.1-36.1%), L-norepinephrine (2.1 20.3%), L-DOPA (6.4-15.8%), and nontreated groups (0-5.6%). Dopamine showed no inducing activity. Treatment with 100 [micro]M carbamylcholine induced 37.6% of metamorphosis in 23-day-old larvae. Larval metamorphosis rate increased significantly with exposure time when treated with acetylcholine, carbamylcholine, and serotonin. Low postlarval survivorship after treatments might nut he related to toxicity of chemicals but due to an energy deficiency after an accelerated completion of metamorphosis in neurochemically stimulated larvae. The effectiveness of inducer drugs was observed to be age-dependent. Nineteen-day old larvae reacted less than 23-day-old ones from the same cohort. Larvae of R. philippinarum responded differently to neurotransmitters from bivalves of the subclass Pterimorphia (i.e., Ostreidae, Pectinidae, Mytilidae) which are more sensitive to catecholamines and L-DOPA. This difference suggests that the mechanisms triggering metamorphosis may differ among bivalve groups.
KEY WORDS: acetylcholine, bivalve, larval age, metamorphosis, neuroactive compounds, postlarval survivorship, Ruditapes philippinarum, serotonin, short-neck clam
INTRODUCTION
Many marine benthic invertebrates produce planktonic larvae. At the end of the planktonic period, larvae respond to complex environmental and biologic stimuli leading to settlement and metamorphosis, allowing the onset of juvenile and adult benthic life forms. This transition is modulated by chemical cues of various biologic origins (Hadfield & Paul 2001). Such cues bind to specific receptors located in the larval neural tissues, triggering metabolic pathways resulting in the behavioral and morphologic changes typical of the metamorphosis process (Burke 1983, Sarojini et al. 1999). Neurotransmitters, their precursors, or similar molecules have been reported to mimic the natural chemical cues and are known to trigger larval settlement and metamorphosis in a variety of marine invertebrates (Hadfield 1984, Morse 1985, Morse 1990, Fires & Hadfield 1991, Okamoto et al. 1995). In bivalves, catecholamines and L-3,4-dihydroxyphenylalanine (L-DOPA) were described as highly effective inducers in Mytilus edulis (Cooper 1982), Crassostrea virginica (Coon et al. 1986), Ostrea edulis and C. gigas (Shpigel et al. 1989), C. belcheri (Tan & Wong 1995), Pecten maximus (Chevolot et al. 1991), and M. galloprovincialis (Satuito et al. 1999). Furthermore, acetylcholine was reported as a settlement inducer for C. gigas (Beiras & Widdows 1995) and gama aminobutyric acid increased the settlement rate of Pinctuda margaritifera (Doroudi & Southgate 2002). Despite their ecological and economical importance, few studies have assessed larval settlement and metamorphosis of clams; soft bottom burrowers like Mercenaria mercenaria (Bachelet et al. 1992, Woodin et al. 1995), Mulinia lateralis (Luckenbach 1984, Grassle et al. 1992), Spisula solidissima (Snelgrove et al. 1998), and Mya arenaria (Snelgrove et al. 1999) have been studied for settlement and habitat selection in terms of hydrodynamics. However, biologic and chemical reguhdion of these processes is largely unknown in clams. We investigated the effects of neuroactive compounds on larval metamorphosis of Ruditapes philippinarum, focusing on a search for highly effective inducers, the postmetamorphic survivorship, and a larval age dependent response. The Japanese short-neck clam is an important commercial resource that sustains fisheries and aquaculture. Recently, disease outbreaks (Hamaguchi et al. 1998, Allam et al. 2000, Renault et al. 2001) along with seasonal (Calvez & Guillou 1998) and environmental fluctuations (Ishii et al. 2001) have diminished wild and farmed populations of this clam. Effective chemicals for induction of larval metamorphosis may improve aquaculture practices and add to our understanding of factors controlling settlement and metamorphosis in clams.
MATERIALS AND METHODS
Larval Cultures
Groups of the Japanese short-neck clam R. philippinarum (total shell length: 27.5 [+ or -] 2.3 mm, n = 40) were collected during reproductive seasons (May and October 2001) from Hamana Bay, Japan (34[degrees]41'33"N, 137[degrees]35'58"E). Spawning was inducted by combining cycles of exposure to cool air (Satuito et al. 1994), immersion in a sperm solution (Loosanoff & Davis 1963) obtained by stripping of 1-2 mature males, and thermal stimulation (Tuba et al. 1994). Finally, the clams were kept individually in 50-mL beakers filled with 1-[micro]m filtered seawater (FSW) at 20[degrees]C for spawning. Released ovules were suspended in FSW in a glass container. Similarly, released sperm was collected and suspended. Adding drops of the sperm solution, a mixture of ovules from several female clams was fertilized. Further on, fertilized zygotes were washed with FSW, placed in 1 L beakers containing fresh FSW, and kept undisturbed for embryonic development at 25[degrees]C (Toha 1992). Straight-hinge veligers were collected 20 to 24 h after fertilization, washed with GF/F-FSW (Whatman GF/F glass-fiber fitters), and placed in 1-L glass containers filled with GF/F-FSW under the following conditions: Initial density of 10 larva/mL (Toba et al. 1994), 25[degrees]C constant temperature (Toba 1992), and salinity of 29-32 p.s.u. throughout the culture period. Aeration was provided for the larval culture containers through glass Pasteur pipettes during the entire culture period. Larval age in days was counted from the first day of larval culture (first straight-hinge veliger). Daily ad libitum food ration was given as follows: An initial diet of Pavlova lutheri at 3 x [10.sup.5] cells/mL (final concentration) was provided until day 5, after which a mixture of Chaetoceros calcitrans and Pavlova lutheri was given at 4 x [10.sup.5] cells/mL until the culture was finished (Tuba et al. 1994). Microalgae for the larval diets were cultured in K medium (Keller & Guillard 1985) at 25[degrees]C and constant illumination. Early pediveligers were observed from days 12 to 14 of culture. Pediveliger culture densities were kept at 1.5 larva/mL. Two experiments were carried out with actively swimming pediveligers. Larval survivorship of the cultures used for experiments 1 and 2 was 24.1% and 31.3%, respectively.
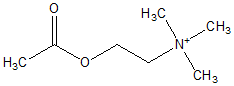
Experiment 1: Effects of Neuroactive Compounds on Larval Metamorphosis
Six neuroactive compounds, acetylcholine chloride (Wako Pure Chemical Industries, Ltd), serotonin creatinine sulfate (Wako), epinephrine bitartrate (Research Biochemicals Inc.), L-norepinephrine bitartrate (Wako), dopamine hydrochloride (Wako) and L-DOPA (Wako) were tested at three concentrations (1, 10, and 100 [micro]M) in triplicate against a control group. Stock solutions were prepared by dissolving in FSW at several folds of the final concentration. Filtered seawater was used fur the control group and dilution of chemical compounds. Eight hundred forty-seven 22-day-old pediveligers were distributed at a density of approximately 1-2 larva/mL in 57 10-mL glass beakers containing FSW. The corresponding solution of neuroactive compound was added to adjust the final concentrations to 1, 10, and 100 [micro]M and final volume to 10 mL in each beaker. Only FSW was added to three of the beakers containing larvae to adjust to the final volume of 10 mL; these were used as control groups. Because oxidation of catechols was expected to occur in seawater, larvae were placed into fresh FSW and newly prepared chemical solutions every 12 h. This procedure was applied to all treatments, subjecting the larvae to the same conditions. Onset of metamorphosis was monitored after 24 and 48 h of exposure. The criteria to determine whether a larva had metamorphosed were by visual detection of postlarval shell growth, complete loss of velum, and observation of the gill rudiment activity using a stereomicroscope and an inverted microscope. Metamorphosed larvae are referred to as postlarvae. After 48 h exposure to the chemicals, larvae and postlarvae were washed, placed in neuroactive-compound-free FSW, and maintained for 24 h to monitor post-treatment survivorship and other effects such as late metamorphosis response. Larvae were not fed during the assay. The experiment was run at 22[degrees]C in a dark, temperature-controlled room at the Fisheries Laboratory of the University of Tokyo, Hamana Bay, in June 2001.
Experiment 2: Effects of Larval Age and Neuroactive Compounds on Larval Metamorphosis
Based on the results obtained in experiment 1, this assay was conducted to evaluate exclusively the cholinergic agonists acetylcholine chloride, carbamylcholine chloride (Nacalai Tesque Inc.), and succinylcholine chloride (Wako), and serotonin creatinine sulfate on the metamorphosis of a cohort of larvae of three age groups, 19, 21, and 23 days old. This assay was run in triplicate against a control group. The concentrations of chemicals were adjusted to 1, 10, and 100 [micro]M and an exposure time of 48 h. A sample of pediveligers was taken from the culture and subjected to the induction assay; remaining siblings were kept in culture until the indicated ages at a density of approximately 1.5 larva/mL and with aeration to avoid settlement. Five hundred fifty 19-day-old larvae and 437 and 339 21- and 23-day-old larvae, respectively, were distributed in 10 mL beakers in a similar way as in experiment 1. Larvae were not fed during the assay. Metamorphosis was monitored after 1, 5, 12, 24, 36, and 48 h of exposure. The trials were run in triplicate against triplicate control groups of FSW under the same conditions as in experiment 1.
Data Analysis
The Kolmogorov-Smirnoff test ([alpha] = 0.05) was used for evaluation of normality. Metamorphosis percentages were analyzed using Kruskal-Wallis analysis of variance (ANOVA) on ranks followed by Dunnet multicomparison ([alpha] = 0.05). The effect of each chemical on larval metamorphosis was classified as positive when the metamorphosis percentage was higher than the control group and as negative when the metamorphosis percentage was equal to or lower than that of the control group. The metamorphosis response at 24 and 48 h in each treatment was compared using a t-test ([alpha] = 0.05). A Kruskal-Wallis ANOVA on ranks followed by a Dunnet multicomparison test ([alpha] = 0.05) was used to analyze the postlarval survivorship among compounds and concentrations. A two-way ANOVA followed by a Tukey test ([alpha] = 0.05) for pairwise comparison was used to assess the effect of larval age on the response to three concentrations of serotonin and choline compounds with the data arcsine square root transformed.
RESULTS
Effects of Neuroactive Compounds on Larval Metamorphosis
Larval metamorphosis of R. philippinarum induced by neuroactive compounds varied with concentration. Positive responses were observed for five of the six chemicals tested (Fig. 1). Acetylcholine at 10 [micro]M showed the highest percentage metamorphosis after 48 h (mean [+ or -] SD n = 3, 92.9 [+ or -] 12.4%) followed by serotonin at 100 [micro]M (80.7 [+ or -] 18.0%) and acetylcholine at 100 [micro]M (70.6 [+ or-] 14.2%). Metamorphosis percentages obtained with these treatments were significantly higher than that of the control group (P < 0.05). Larvae exposed to either 1 [micro]M acetylcholine or 1 [micro]M serotonin did not metamorphose during the 48-h assay period. Positive chemicals showed no differences in induction activities at 24 h and 48 h of exposure (P > 0.05), except for acetylcholine at 10 and 100 [micro]M (P < 0.001 and P = 0.005, respectively), where the metamorphosis was induced within 24 h. The metamorphosis percentage was significantly increased when larvae were exposed to higher concentrations of acetylcholine (P = 0.021) and serotonin (P = 0.028). Although epinephrine showed a positive effect, there were no statistical differences between epinephrine and the control group (P = 0.166). Larvae of R. philippinarum metamorphosed in response to L-norepinephrine and L-DOPA, yet they were less effective, yielding low percentage metamorphosis, and were not significantly different from the control group. Dopamine showed no inductive effect at all concentrations tested after 48 h of exposure. Larval mortalities were observed when exposed to dopamine and L-DOPA at concentrations of 100 and 10 [micro]M.
[FIGURE 1 OMITTED]
Larvae exposed to the effective chemicals changed their regular swimming pattern, combining crawling and short swimming near the bottom. A progressive extension of the crawling period was observed to be dependent on exposure time. Vela in the pediveligers lost most of their cilia. When there was no movement, we assumed they were metamorphosing, because larvae and postlarvae always crawled before and after metamorphosis; no byssal attachment was observed.
Effects of Neuroactive Compounds on Postlarval Survivorship
After assays, larvae and postlarvae were transferred to fresh FSW to observe post treatment responses after 24 h. Postlarval survivorships are summarized in Table 1. Variability was observed in the postlarval survival rate after exposure to neurochemicals for 48 h. All postlarvae died after exposure to L-DOPA in all concentrations tested. Larvae that did not undergo metamorphosis died in all cases studied, except for the control group and the 1 [micro]M acetylcholine, 1 [micro]M serotonin, and 1 [micro]M dopamine treatments, where remaining pediveligers displayed active swimming or crawling behavior. In these trials, newly metamorphosed larvae were observed (acetylcholine, 4.3 [+ or -] 3.8%; serotonin, 14.2 [+ or -] 1.6%; and dopamine, 7.8 [+ or -] 6.9%) and recorded as post-treatment metamorphosis (Table 1). No significant differences (P = 0.944) in post larval survivorships at the concentrations tested were observed among treatments. This result indicates that concentrations of 1, 10, and 100 [micro]M of the neurochemicals used for metamorphosis induction may not have affected the postlarval survivorship, except for treatments with L-DOPA at the higher concentrations, which led to 100% postlarval mortalities. The percentage survivorship of postlarvae after the treatments with acetylcholine, serotonin, epinephrine, and L-norepinephrine was not significantly different from that of the control group (100% survival) (P = 0.062).
Effects of Larval Age and Neuroactive Compounds on Larval Metamorphosis
Ruditapes philippinarum pediveligers of the three age groups (19, 21, and 23 days old) from the same cohort showed a distinct metamorphosis rate when exposed to various concentrations of choline derivatives and serotonin (Fig. 2). The highest metamorphosis percentages were obtained when 21-day-old larvae were exposed to 10 [micro]M serotonin and 23-day-old larvae to 10 and 100 [micro]M serotonin (mean [+ or -] SD n = 3, 71.4 [+ or -] 49.5%, 61.8 [+ or -] 18.6%, and 64.0 [+ or -] 10.3%, respectively), of which only the responses of 23-day-old larvae to 10 and 100 [micro]M serotonin were significantly higher than the control group (P < 0.05). Lower metamorphosis percentages yet significantly different from control groups were observed in 21-day-old larvae treated with 1 and 100 [micro]M acetylcholine and 100 [micro]M carbamylcholine (P < 0.05). Percentage metamorphosis of 23-day-old larvae treated with acetylcholine 100 [micro]M was also significant (P < 0.05). Among age groups, metamorphosis percentages obtained when exposed to FSW (control group) were not significantly different (P = 0.089). The larval age was found to influence significantly larval metamorphosis induced by acetylcholine (P < 0.001), carbamylcholine (P < 0.001), and serotonin (P = 0.011). Younger larvae exhibited the lower response to the chemicals. A significant increase in the percentage metamorphosis was observed when 19-day-old larvae were compared with 23-day-old larvae (P < 0.05). Responses of 21- and 23-day-old larvae to acetylcholine, carbamylcholine, and serotonin were not significantly different (P > 0.05). Metamorphosis rate did not increase with exposure time when larvae of the 19-day-old group were stimulated with neuroactive compounds (P = 0.059, Fig. 3). A significant time dependent response was observed for larvae of 21-day-old (P = 0.003) and 23-day-old (P < 0.001) when treated with acetylcholine, carbamylcholine, and serotonin. It should be noted that the onset of metamorphosis was observed alter 5-h exposure for 23 day-old larvae, which subsequently metamorphosed within 24 h. Percentage metamorphosis did not significantly increase for concentrations of serotonin (P = 0.331). acetylcholine (P = 0.123), and carbamylcholine (P = 0.175). Acetylcholine and carbamylcholine were effective at lower and higher concentrations. Similar trends were observed for acetylcholine in experiment 1. Serotonin was more effective at higher concentrations for older larvae. Effectiveness of serotonin at the highest concentration tested was observed in experiment 1 as well. However, the percentage metamorphosis of 21 day-old larvae when exposed to 100 [micro]M serotonin was similar to that of the control group (P = 0.269). This outcome was in contrast to the effectiveness of 100 [micro]M serotonin observed in experiment 1 and later with older sibling larvae. Succinylcholine exhibited the least effect; no significant differences among age groups and doses were observed (19-day-old, P = 0.392: 21-day-old, P = 0.392; 23-day-old, P = 0.278).
[FIGURES 2-3 OMITTED]
DISCUSSION
Effects of Neuroactive Compounds on Larval Metamorphosis
Our investigation demonstrated that the neuroactive compounds serotonin and acetylcholine induced larval metamorphosis of the Japanese short-neck clam R. philippinarum. We first tried to identify highly effective inducer neuroactive compounds in terms of high postlarval yield. We assessed different types of neuroactive compounds: the choline derivative acethylcholine, the indolamine serotonin, and the catechols epinephrine, L-norephinephrine, dopamine, and L-DOPA. Among the compounds tested, acetylcholine (10 and 100 [micro]M) and serotonin (100 [micro]M) exhibited the highest inducing activity on larval metamorphosis. Soon after exposure to these chemicals, pediveligers exhibited a behavioral shift from swimming to crawling actively on the bottom. Most larvae completed metamorphosis in 48 h. In contrast, only weak activity of serotonin at the same concentration ranges was reported for Mytilus galloprovincialis (Satuito et al. 1999), Crassostrea gigas (Beiras & Widdows 1995), and Patinopecten yessoensis (Kingzett et al. 1990). Acetylcholine was previously reported to he ineffective for M. galloprovincialis (Satuito et al. 1999), but an "active" inducer for C. gigas, though only 20-40% of larvae metamorphosed (Beiras & Widdows 1995). Recently, it was reported that acetylcholine induced <40% metamorphosis in Mytilus edulis larvae (Dobretsov & Qian 2003). Interestingly, the catecholamines, epinephrine and norepinephrine, and L DOPA were not effective inducers of larval metamorphosis of R. philippinarum. The ineffectiveness of epinephrine and norepinephrine was also observed for the Venus clam Ruditapes largillierti (Kent et al. 1999). Thus, clam species of the genus Ruditapes (subclass Heterodonta) seem to exhibit a different response to neuroactive compounds than bivalves in the subclass Pterimorphia (i.e., Ostreidae, Pectinidae, Mytilidae), which have routinely been induced to metamorphose by epinephrine, norepinephrine, or L-DOPA. Our results suggest that acetylcholine and serotonin may play roles in the metamorphosis of R. philippinarum. Regardless of the internal regulatory mechanism, solutions of these neuroactive compounds accelerated metamorphogenic changes in R. philippinarum pediveligers. In the absence of an appropriate chemical inducer, metamorphosis did occur, but at a slower rate. as was observed in the pediveligers incubated in FSW.
Although there are differences in chemicals capable of inducing larval metamorphosis in R. philippinarum, behavioral and morphologic changes associated with the end of larval life were rather similar to bivalves reported in other studies. R. philippinarum exhibited a change of the typical pediveliger swimming behavior; the larva showed alternating cycles of near-bottom swimming and crawling followed by only crawling on the substrate until the onset of metamorphosis, where no motion was observed until the completion of the final anatomic changes of metamorphosis. However, R. philippinarum pediveligers do not attach to the substrate prior to metamorphosis; they do nut secret a cementing or fixing substance unlike oysters (Cranfield 1973), mussels (Eckroat & Steele 1993). and scallops (Benninger & Le Pennec 1991, Tapia et al. 1993).
Effects of Neuroactive Compounds on Postlarval Survivorship
Postlarvae of R. philippinarum obtained by treatment with neuroactive compounds had a very variable and low survival rate after treatments. We observed that the survival rate did not differ significantly among all concentrations, but it did differ among compounds. Dopamine and L-DOPA at higher concentrations appeared to be toxic after 48 h of exposure. When treated with acetylcholine, serotonin, epinephrine, and L-norepinephrine, the postlarval survival rate was low. The control group exhibited a remarkable survivorship of 100%. In contrast, with treatments of acetylcholine and serotonin, a much higher percentage of metamorphosed larvae was observed within 48 h of exposure, yet surprisingly, a high proportion of those postlarvae was not able to survive. Primarily, this low survival rate seems to be related to the chemical treatment (i.e., a toxicity degree). However, taking into account that our assay was carried out under no feeding conditions---exposing the individuals to over 72 h of starvation the low postmetamorphosis survivorship may be due to an energy deficiency rather than toxicity of neuroactive compounds. Treatments of effective inducers such as acetylcholine and serotonin may have intervened the neural pathways needed to trigger metamorphosis, accelerating the process (Hadfield 2000). Consequently, more larvae underwent metamorphosis prior than they could replete metabolic reserves to endure metamorphosis. This may explain our low postlarval survival rate under nonfeeding conditions. However, the fact that 100% survival of non-fed "spontaneously" metamorphosed postlarvae in the control groups impedes reconciling of our results. For this reason, our arguments need to be addressed in future studies. Mortality rates after induction of metamorphosis by chemicals have also been reported for oysters. Haws et al. (1993), in the oysters C. gigas and (2 virginica, found an increment of mortality due to a depletion of lipids, carbohydrates, and proteins 36 h after larval metamorphosis induced by epinephrine. Interestingly, Shpigel et al. (1989) reported no negative effects of epinephrine on the survival rate of O. edulis cultchless spats fed daily with a mixed microalgae diet. Consequently, food availability after metamorphosis may be one of the main factors determining postlarval survivorship. Therefore, effective neuroactive compounds may serve as postlarval yield enhancers only when the food supply after the treatment is sufficient.
Effects of Larval Age and Neuroactive Compounds on Larval Metamorphosis
We demonstrated that larval age had a significant effect on larval response to effective neuroactive compounds. The effectiveness of acetylcholine and serotonin was low in 19-day-old larvae treated for 48 h. Sibling larvae only 2 days older exhibited a significantly enhanced response to the same chemicals. Among the older larvae assayed in our experiments (21 and 23 days old), the responses to the effective neuroactive compounds were similar. Thus, it seems that effective neuroactive compounds such as serotonin and acetylcholine have the same effect on metamorphosis of older larvae. Taken together, in the results of both experiments we find support to conclude that R. philippinarum pediveligers older than 21 days become competent to metamorphosis in response to acetylcholine and serotonin. The same pattern was observed when larvae were exposed to carbamylcholine, an acetylcholine receptor agonist. This compound exhibited a similar activity-increment to that observed with acetylcholine in the same range of concentrations, though it was less effective. These findings along with those obtained in experiment 1 may suggest that acetylcholinergic and serotoninergic neural pathways play roles in triggering metamorphosis in R. philippinarum. The observed age-dependency of the clam's responsiveness to chemical-inducing stimuli may suggest the development of a sensory system at the end of the pediveliger stage for the recognition of specific cues for settlement and metamorphosis (Hirata & Hadfield 1986, Pawlik 1992, Hadfield et al. 2000). We assumed that the effective neuroactive compounds stimulated those receptors directly involved in metamorphosis regulation of the short-neck clam. A great deal of variability in the larval responses to neuroactive compounds was evident, which may be explained in part by the variability in the degree of development among individuals in the cohort. The effectiveness of serotonin and acetylcholine al various larval ages suggests that these compounds could be used to synchronize several larval cohorts for postlarval yield. These may have useful applications in aquaculture production as well as in metamorphosis studies of the Japanese short neck clams.
ACKNOWLEDGMENTS
The authors thank the anonymous reviewers for their valuable comments on the manuscript.
LITERATURE CITED
Allam, B., C. Paillard, A. Howard & M. Le Pennec. 2000. Isolation of the pathogen Vibrio tapetis and defense parameters in brown ring diseased Manila clams Ruditapes philippinarum cultivated in England. Dis. Aquat. Org. 41:105-113.
Bachclet, G., C. A. Butman, C. M. Webb, V. R. Starczak & P. V. R. Snelgrove. 1992. Non-selective settlement of Mercenaria mercenaria (L.) larvae in short-term, still-water, laboratory experiments. J. Exp. Mar. Biol. Ecol. 161:241-280.
Beiras, R. & J. Widdows. 1995. Induction of metamorphosis in larvae of the oyster Crasssotrea gigas using neuroactive compounds. Mar. Biol. 123:327-334.
Benninger, P. G. & M. Le Pennec. 1991. Functional anatomy of scallops. In: S. E. Shumway, editor. Scallops: biology, ecology and aquaculture. Amsterdam: Elsevier. pp. 204 208.
Burke, R. D. 1983. The induction of metamorphosis of marine invertebrate larvae: stimulus and response. Can. J. Zool. 61:1701-1719.
Calvez, I. & J. Guillou. 1998. Impact of winter mortalities on postlarval and juvenile stages in Ruditapes philippinarum from western Brittany. J. Mar. Biol. Assoc. U.K. 78:1381-1384.
Chevolot, L., J. C. Cochard & J. C. Yvin. 1991. Chemical induction of larval metamorphosis of Pecten maximus with a note on the nature of the naturally occurring triggering substances. Mar. Ecol. Prog. Ser. 74:83-89.
Coon, S. L., D. B. Bonar & R. M. Weiner. 1986. Chemical production of cultchless oyster spat using epinephrine and norepinephrine. Aquaculture 58:255-262.
Cooper, K. 1982. A model to explain the induction of settlement and metamorphosis of planktonic eyed-pediveligers of the blue mussel Mytilus edulis by chemical and tactile cues. J. Shellfish Res. 2:117.
Cranfield, H. J. 1973. Observations on the behavior of the pediveliger of Ostrea edulis during attachment and cemmenting. Mar. Biol. 22:203 209.
Dobretsov, S, V. & P. Y. Qian. 2003. Pharmacological induction of larval settlement and metamorphosis in the blue mussel Mytilus edulis L. Biofouling 19:57-63.
Doroudi, M. S. & P. C. Southgate. 2002. The effect of chemical cues on settlement behaviour of blacklip pearl oyster (Pinctada margaritifera) larva. Aquaculture 209:117-124.
Eckroat, L. R. & L. M. Steele. 1993. Comparative morphology of the byssi of Dreissena polymorpha and Mytilus edulis. Am. Malacol. Bull 10: 103-108.
Grassle, J. P., P. V. R. Snelgrove & C. A. Butman. 1992. Larval habitat choice in still water and flume flows by the opportunistic bivalve Mulinia lateralis. Neth. J. Sea Res. 30:33-44.
Hadfield, M. G. 1984. Settlement requirements of molluscan larvae: new data on chemical and genetic roles. Aquaculture 39:283-298.
Hadfield, M. G. 2000. Why and how marine-invertebrate larvae metamorphose so fast. Cell Dev. Biol. 11:437-443.
Hadfield, M. G., E. A. Meleshkevitch & D. Y. Boudko. 2000. The apical sensory organ of a gastropod veliger is a receptor for settlement cues. Biol. Bull 198:67-76.
Hadfield, M G. & V. J. Paul. 2001, Natural chemical cues for settlement and metamorphosis on marine-invertebrate larvae. In: J. B. McClintock & B. J. Baker, editors. Marine chemical ecology. Boca Raton: CRC Press. pp. 431-161.
Hamaguchi, M., N. Suzuki H. Usuki & H. Ishioka. 1998. Perkinsus protozoan infection in short-necked clam Tapes (=Ruditapes) philippinarum in Japan. Fish Pathol. 33:473-480.
Haws, M. C., L. DiMichele & S. C. Hand. 1993. Biochemical changes and mortality during metamorphosis of the eastern oyster Crassostrea virginica, and the pacific oyster Crassostrea gigas. Mol. Mar. Biol. Biotechnol. 2:207-217.
Hirata, K. Y, & M. G. Hadfield. 1986. The role of choline in metamorphic induction of Phestilla (Gastropoda, Nudibranchia). Comp. Biochem. Physiol. 84C:15-21.
Ishii, R., H. Sekiguchi, Y. Nakayama & Y. Jinnai. 2001. Larval recruitment of the manila clam Ruditapes philippinarum in Ariake Sound, southern Japan. Fisheries Sci. 67:579-591.
Keller, M. D. & R. Guillard. 1985. Factors significant to marine dinoflagellate culture. In: D. M. Anderson. A. W. White & D. G. Baden, editors. Toxic dinoflagellates. New York: Elsevier Science. pp. 113-116.
Kent, G. N., G. B. Maguire, I. Duthie & R. Pugh. 1999. Spawning, settlement and growth of the New Zealand venerid Ruditapes largillierti (Philippi 1849) in culture. N. Z. J. Mar. Freshwater Res. 33:55-62.
Kingzett, B. C., N. Bourne & K. Leask. 1990. Induction of metamorphosis of the Japanese scallop Patinopecten yessoensis Jay. J. Shellfish Res. 9:119-126.
Loosanoff, V. L. & H. C. Davis. 1963. Rearing of bivalve mollusks. Adv. Mar. Biol. 1:1-136.
Luckenbach, M. W. 1984. Settlement and early post settlement survival in the recruitment of Mulinia lateralis (Bivalvia). Mar. Ecol. Prog. Ser. 17:245-250.
Morse, D. E. 1985. Neurotransmitter-mimetic inducers of larval settlement and metamorphosis. Bull. Mar. Sci. 37:697-706.
Morse, D. E. 1990. Recent progress in larval settlement and metamorphosis: closing the gaps between molecular biology and ecology. Bull. Mar. Sci. 46:465-483.
Okamoto, K., A. Watanabe, N. Watanabe & K. Sakata. 1995. Induction of larval metamorphosis in serpulid polychaetes by L-DOPA and cathecolamines. Fisheries Sci. 61:69-74.
Pawlik, J. R. 1992. Chemical ecology of the settlement of benthic marine invertebrates. Oceangr. Mar. Biol. Annu. Rev. 30:273-335.
Pires, A. & M. G. Hadfield. 1991. Oxidative breakdown products of catecholamines and hydrogen peroxide induce partial metamorphosis in the nudibranch Phestilla sibogae Bergh (Gastropoda: Opisthobranchia). Biol. Bull. 180:310-317.
Renault, T., C. Lipart & I. Arzur. 2001. A herpes like virus infecting Crassostrea gigas and Ruditapes phillipinarum larvae in France. J. Fish Dis. 24:369-376.
Sarojioi, R., R. Nagabhushanam & M. Fiugerman. 1999. Induction of larval settlement and metamorphosis by neuroactive compounds in marine invertebrates. In: M. Fingerman, R. Nagabhushanam & M. F. Thompson, editors. Recent advances in marine biotechnology, vol. 3: biofilms, bioadhesion, corrosion, and biofouling. Enfield: Science Publishers, pp. 203-222.
Satuito, C. G., K. Natoyama, M. Yamazaki & N. Fusetani, 1994. Larval development of the mussel Mytilus edulis galloprovincialis cultured under laboratory conditions. Fisheries Sci. 60:65-68.
Satuito, C. G., K. Natoyama, M. Yamazaki, K. Shimizu & N. Fusetani. 1999. Induction of metamorphosis in the pediveliger larvae of the mussel Mytilus galloprovincialis by neuroactive compounds. Fisheries Sci. 65:384-389.
Shpigel, M., S. L. Coon & P. Kleinot. 1989. Growth and survival of cultchless spat of Ostrea edulis Linneus, 1750 produced using epinephrine and shell chips. J. Shellfish Res. 8:355-357.
Snelgrove, P. V. R., J. P. Grassle & C. A. Butman. 1998. Sediment choice by settling larvae of the bivalve, Spisula solidissima (Dillwyn), in flow and still water. J. Exp. Mar. Biol. Ecol. 231:171-190.
Snelgrove, P. V. R., J. Grant & C. A. Pilditch. 1999. Habitat selection and adult-larvae interactions in settling larvae of sorer-shell clam Mya arenaria. Mar. Ecol. Prog. Ser. 182:149-159.
Tan, S. H. & T. M. Wong. 1995, Induction of settlement and metamorphosis in the tropical oyster. Crassostrea belcheri (Sowerby), by neuroactive compounds. J. Shellfish Res. 14:435-438.
Tapia, C., E. Dupre & G. Bellolio. 1993. Descripcion del comportamiento de asentamiento larval de pediveligeras de Argopecten purpuratus Lamark 1819. Rev. Biol. Mar. 28:75-84.
Toba, M. 1992. Relationship between temperature and larval survival growth rate in Manila clam Ruditapes philippinarum. Bull. Chiba Pref. Exp. Sta. 50:17-20.
Toba, M., Y. Miyama & M. Sakai. 1994. Mass culture of the Isochrysis aff. galvuna-IV food value to the spat of the manila clam Ruditapes philippinarum led as single-specie algal diet. Saibai Giken 22:75-81.
Woodin, S. A., S. M. Lindsay & D. S. Wethey. 1995. Process-specific recruitment cues in marine sedimentary systems. Biol. Bull. 189:49-58.
PAULA M. URRUTIA, (1) KEN OKAMOTO (2) AND NOBUHIRO FUSETANI (1) * (1) Laboratory of Aquatic Natural Products Chemistry, Department of Aquatic Biosciences, and (2) Laboratory of Aquatic Conservation, Department of Ecosystem Studies, Graduate School of Agricultural and Life Sciences, The University of Tokyo, Bunkyo-ku, Tokyo 113-8657, Japan
* Corresponding author. E-mail: anobu@mail.ecc.n tokyo.ac.jp
COPYRIGHT 2004 National Shellfisheries Association, Inc.
COPYRIGHT 2005 Gale Group