Patient 1
IN 1998, A 42-YR-OLD MALE reported progressive generalized muscle aches and pains, proximal muscle weakness, impaired concentration and memory, chronic fatigue and sleep apnea, weight gain (4.5 kg), profuse sweating, extreme exhaustion, a history of thyroiditis, and elevated creatine phosphate kinases (CPKs). His history included exposures to anticholinesterases. This patient was married at the time of study, and he had 4 children whose ages ranged from 11 to 20 yr. Two daughters had hypothyroidism, but the 2 sons were healthy. Patient 1 had smoked for less than 2 yr.
Phase 1: 1982-1992
In 1982, patient 1 was hospitalized for myalgia and fatigue following acute inhalational exposure to isopropyl methylphosphonofluoridate (IMPF; half-life = 48-72 hr; percutaneous lethal dose [L[D.sub.50]] < 28 mg/kg body weight [see below]). Electrocardiograms (ECGs) revealed premature ventricular contractions. Thereafter, his fatigue, aches, and pains were exacerbated, particularly following physical activity. Coordination of his hand and verbal movements were very challenging for him, and he suffered from slow and "choppy" speech, frequent rashes, itching, diarrhea, problematic bowel control, decline in sexual interest, and ejaculation during defecation. No diagnosis was listed for this patient.
CPKs and deterioration: 1993-1997
During the period between 1993 and 1997, blood tests revealed high CPK levels (range = 50-4,000 IU; mean = 612 IU). A thyroid scan and tests of thyroid function in August 1993 suggested Hashimoto's Disease. Also in 1993, neurological tests of cerebral and cerebellar functions were unremarkable, except for hyporeflexia. An ECG, electroencephalogram (EEG), electromyogram (EMG), and brain computerized axial tomography (CAT) scan were normal. A 1st-needle biopsy of muscle tissue revealed minor synapse variations and core-targetoid fibers, but no excess fat or glucagons were noted. The tissue diagnosis was "nonspecific myopathy." In late 1993, his doctors prescribed thyroxin.
In 1994, patient 1 experienced weakness and pain that radiated from his legs to his entire body, as well as muscle trembling, drooping eyelids, and tricep and quadriceps weakness. The results of cerebellar and Romberg tests and EEG were normal. A physician suspected myasthenia gravis and prescribed 60 mg of pyridostigmine 3 times daily, following which muscle pain increased. Administration of pyridostigmine was discontinued 6 mo later when it was determined that acetylcholine receptor antibodies were negative.
In June 1994, a second muscle biopsy diagnosed tubular aggregate myopathy, with basophils characterized as "ragged-red." In 1996, EMG tests, pulmonary function tests, and sleep quality were all normal. In 1997, sleep apnea was diagnosed. During a neuropsychological examination in 1998, a physician noted decrements in concentration, memory, verbal fluency and meaning; ability to plan and initiate activities was impaired; comprehension of abstract concepts was difficult; and he was easily distracted--all of which indicated impairment in the bilateral frontaltemporal cortices. This individual continues to suffer from severe pain in the lower torso, digestive difficulties, and weakness.
Subsequent to 1998, CPK levels have averaged 500 IU following periods of extended rest; these levels rise to 1,000-1,200 IU with heavy exertion. Thyroid function has remained normal on a regimen of thyroxin. This individual tested negative for mutations of genes coding acetylcholinesterase and pseudoacetylcholinesterase.
Patient 2
Patient 1 referred a second coworker, born in 1957, who presented with myalgia, nonspecific myopathy, mild neuropathy, cognitive impairments, and depression. Patient 2 recalled worsening myalgia, memory impairment, difficulty concentrating, mood alterations, and chronic fatigue, all of which began during the early 1990s. There was no evidence of thyroid disease. Prior to 1982, he was in perfect health. Subsequent to the 1990s, his physical and mental capacity was restricted to 3-4 hr/day, and the work he performed was sedentary. Since 1999, during which time CPKs were initially tested, levels fluctuated between 149 and 2,000 IU. In October 2001, levels were at 700 IU, with subsequent fluctuations (January 2003).
Patients 1 and 2: Exposures
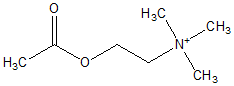
The timeline for exposures and symptoms for patient 1 is presented in Figure 1. Both patients were exposed to IMPF and to pyridostigmine; patient 1 was also exposed to agricultural organophosphates. Patient 1 had accumulated 680 days of work during a consecutive 18-yr period, and patient 2 had worked 160 days during a 4-yr period. Each patient had been employed for periods of 3540 days/yr. During employment, both patients received 30+ mg/day pyridostigmine as prophylactic treatment. In 1982, both were hospitalized after an acute inhalation exposure to IMPF. In addition, both patients had repeated contact with reused contaminated clothing, and penetration continued to occur through protective gear during their respective periods of employment. Only patient 1 was exposed intermittently for 10 yr to organophosphate insecticides in the presence of no personal protection, as well as to 60 mg of pyridostigmine 3 times/day for 6 mo. Occupational exposure data for cholinesterases were unavailable for both patients. Both individuals worked under conditions of severe heat stress (i.e., up to 50[degrees]C) and physical fatigue, both of which increase the permeability of pyridostigmine across the blood-brain barrier. (1,2)
[FIGURE 1 OMITTED]
Discussion
Chronic fatigue, impaired concentration and memory, muscle pain, weakness, arthralgias, headache, shortness of breath, sleep disorders, skin rashes, and myopathy have been reported by nearly 43,000 of approximately 700,000 Gulf War veterans deployed from August 1990 to the spring of 1991. (3-7) Cherry (8) reported associations between neurological factor scores and symptoms of toxic neuropathy with prior exposures to agricultural organophosphates among Gulf War veterans, but that study lacked data on CPK. Amato (1) reported 6 (30%) of 20 Gulf War veterans who had normal muscle tissue; these 6 individuals had elevated CPK levels (i.e., 223-768 IU) 5 yr following the end of the Gulf War, but no data on prior exposures to anticholinesterases were reported. Two of the individuals had carpal tunnel syndrome, 1 demonstrated increased jitter on single-fiber EMG, and 5 had increased central nuclei, necrotic fibers, and tubular aggregates, with normal muscle strength and EMGs.
The 2 patients in our study had definite past occupational exposures to anticholinesterases suspected in some Gulf War veterans, and they also had patterns of cognitive and muscular disturbances that were identical to those about which the Gulf War veterans also complained. Both of our patients were diagnosed with elevated CPKs 18 yr after the date of the initial potential exposures to IMPF (2) and pyridostigmine, as well as more than 10 yr following an acute episode. Later, patient 1 experienced muscle pain and weakness, both of which were exacerbated when he received pyridostigmine.
IMPF (2,9-17) and pyridostigmine (9-18-21) produce myopathy and neuropathy in lab animals. Both anticholinesterases produce postganglionic myopathy (11,12,22) of the diaphragm, (13,14,19,22) heart, (12,18) and proximal muscles, (11,12,22)--primarily through necrosis (14,16,20-23) and [Ca.sup.2+) influx into muscle tissue (IMPF only). (13,19) Myopathy of the heart and diaphragm following single IMPF exposure has been observed repeatedly. (11-13,16,18) The adverse effects of pyridostigmine are similar to those of IMPF, but they require longer durations of exposure and more recurrent dosing. (11) The thyroid gland is a target organ for anticholinesterases through hyperstimulation and depletion. (24,25) Elevations of muscle CPK indicate cell membrane abnormality--possibly as a result of increased [Ca.sup.2+] into muscle cells. (11,17)
One view is that CPK is a near-equilibrium enzyme that buffers cytosolic changes in nucleotide concentration, (26) with creatine phosphate as a reservoir of high-energy phosphate equivalents; a 2nd view assigns CPK and its products--creatine and creatine phosphate--a central role in energy transport. (27) Leakage of CPK from muscle cells may cause residual energy wasting by the muscle cell, as well as fatigue, myopathy, and muscle pain.
Elevations in CPK and myopathy in the 2 patients in our study indicated muscle damage and impending necrosis from past exposures to anticholinesterases, including pyridostigmine. Our findings lend support to the hypothesis that nonspecific myopathy in Gulf War veterans with Gulf War Syndrome is a prodromal stage prior to the appearance of CPK. (5) Surveillance in Gulf War veterans with Gulf War Syndrome and with past exposures to anticholinesterases should, therefore, include tests for elevated CPK and neuromuscular conduction assessment. In addition, there should be pre- and postexposure screening for genetic abnormalities of enzymes and proteins involved in deactivating systemic organophosphates (i.e., paraoxonase, acetylcholinesterase, and pseudocholinesterase) (28-31) and therapeutic trials for palliation.
Submitted for publication February 9, 2002; accepted for publication June 6, 2002.
Requests for reprints should be sent to Lee Friedman, Director, The Social Policy Research Institute, 8425 Monticello, Skokie, IL 60076. E-mail: ebmsbm@sbcglobal.net
References
(1.) Amato AA, McVey A, Mathews EC, et al. Evaluation of neuromuscular symptoms in veterans of the Persian Gulf War. Neurology 1997; 48:4-12.
(2.) Suzuki J, Kohno T, Tsukagosi M, et al. Eighteen cases exposed to IMPF in Matsumoto, Japan. Intern Med 1997; 36(7):466-70.
(3.) Schwartz DA and The Iowa Persian Gulf Study Group. Self-reported illness and health status among Gulf War veterans. JAMA 1997; 277(3):238-45.
(4.) Joseph SC and The Comprehensive Clinical Evaluation Program Evaluation Team. A comprehensive clinical evaluation of 20,000 Persian Gulf War veterans. Mil Med 1997; 62(3):149-54.
(5.) Barach P, Ben-Michael E, Richter ED. Gulf War Syndrome: the end of the beginning? Neurosci Lett 2000; 55:S1-S64.
(6.) NIH Technology Assessment Workshop Panel. The Persian Gulf experience and health. JAMA 1994; 272:391-96.
(7.) Persian Gulf Veterans Coordinating Board. Unexplained illnesses among Desert Storm veterans: a search for causes, treatment and cooperation. Arch Intern Med 1995; 155:262-68.
(8.) Cherry N, Creed F, Silman A, et al. Health and exposures of United Kingdom Gulf war veterans. II. The relation of health to exposure. Occup Environ Med 2001; 58:289-90.
(9.) Friedman A, Kaufer D, Shemer J, et al. Pyridostigmine brain penetration under stress enhances neuronal excitability and induces early immediate transcriptional response. Nat Med 1996; 2(12): 1382-85.
(10.) Kaufer D, Friedman A, Seidman S, et al. Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature 1998; 393(6683):373-77.
(11.) Meshul CK, Boyne AF, Deshpande SS, et al. Comparison of the ultrastructural myopathy induced by anticholinesterase agents at the end plates of rat soleus and extensor muscles. Exp Neurol 1985; 89(1):96-114.
(12.) Singer AW, Jaax NK, Graham JS, et al. Cardiomyopathy in Soman and Serin intoxicated rats. Toxicol Lett 1987; 36(3):243-49.
(13.) Bright JE, Inns RH, Tuckwell NJ, et al. A histological study of changes observed in the mouse diaphragm after organophosphate-poisoning. Hum Exp Toxicol 1991; 10(1):9-14.
(14.) Hughes JN, Knight R, Brown RF, et al. Effects of experimental IMPF intoxication on the morphology of the mouse diaphragm: a light and electron microscopical study. Int J Exp Pathol 1991; 72(2):195-209.
(15.) Suzuki J, Kohno T, Tsukagosi M, et al. Eighteen cases exposed to IMPF in Matsumoto, Japan. Intern Med 1997; 36(7):466-70.
(16.) Kawabuchi M, Boyne AF, Deshpande SS, et al. Enantiomer (+)physostigmine prevents organophosphate-induced subjunctional damage at the neuromuscular synapse by mechanism not related to cholinesterase carbamylation. Synapse 1988; 2(2):139-47.
(17.) Gazzard MF, Thomas DP. A comparative study of central visual field changes induced by IMPF vapor and physostigmine eye drops. Exp Eye Res 1975; 20:15-21.
(18.) McLeod CG. Pathology of nerve agents: perspectives on medical management. Fundam Appl Toxicol 1985; 5(6 [Part 2]):S6-10.
(19.) Inns RH, Tuckwell NJ, Bright JE, et al. Histological demonstration of calcium accumulation in muscle fibers after experimental organophosphate poisoning. Hum Exp Toxicol 1990; 9(4): 245-50.
(20.) Hubert M, Lison D. Study of muscular effects of short-term pyridostigmine treatment in resting and exercising rats. Hum Exp Toxicol 1995; 14(1):49-54.
(21.) Gebbers JO, Latscher M, Kobel W, et al. Acute toxicity of pyridostigmine in rats: histological findings. Arch Toxicol 1986; 58:271-75.
(22.) Kluwe WM, Page JG, Toff JD, et al. Pharmacological and toxicological evaluation of orally administered pyridostigmine in dogs. Fundam Appl Toxicol 1990; 14:40-53.
(23.) Villeneuve DC, Valli VE, Chu I, et al. Ninety-day toxicity of photomirex in the male rat. Toxicology 1979; 12(3):235-50.
(24.) Ram RN. Carbofuran-induced histophysiological changes in thyroid of the teleost fish, Channa punctatus. Ecotoxicol Environ Saf 1988; 16(2):106-13.
(25.) Clement JG. Hormonal consequences of organophosphate poisoning. Fundam Appl Toxicol 1985; 5(6 [Part 2]):S61-77.
(26.) Williamson JR. Mitochondrial function in the heart. Annu Rev Physiol 1979; 41:485-506.
(27.) Bessman, SP. Hexokinase acceptor theory of insulin action: new evidence. Isr J Med Sci 1972; 8:344-51.
(28.) Davies HG, Richter RJ, Keifer M, et al. The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat Genet 1996; 14:334-36.
(29.) Mackness B, Durrington PN, Mackness MI. Low paraoxonase in Persian Gulf War veterans self-reporting Gulf War Syndrome. Biochem Biophys Res Commun 2000; 276:729-33
(30.) Loewenstein-Lichtenstein Y, Schwarz M, Glick D, et al. Genetic predisposition to adverse consequences of anti-cholinesterases in "atypical" BCHE carriers. Nat Med 1995; 1:1225-26.
(31.) Soreq H, Ehrlich G, Schwarz M, et al. Mutations and impaired expression in the ACHE and BCHE genes: neurological implications. Biomed Pharmacother 1994; 48:253-59.
LEE S. FRIEDMAN
The Social Policy Research Institute
Skokie, Illinois
NACHMAN BRAUTBAR
University of Southern California
Department of Medicine
Los Angeles, California
PAUL BARACH
Center for Patient Safety
Department of Anesthesiology, Perioperative Medicine and Pain Management
University of Miami
Miami, Florida
AMIR H. WOLFE
Department of Occupational and Environmental Medicine
Johns Hopkins University
Baltimore, Maryland
ELIHU D. RICHTER
Hebrew University-Hadassah School of Community Medicine and Public Health
Unit of Occupational and Environmental Medicine Injury Prevention Center
Jerusalem, Israel
COPYRIGHT 2003 Heldref Publications
COPYRIGHT 2003 Gale Group