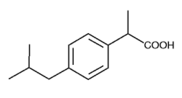
Whitehall-Robins Healthcare now has an easy-to-hold bottle and "arthritis friendly" cap for its 165-count Advil pain reliever.
Introduced in the first quarter of 2001, the new packaging for tablets, caplets and gel caplets is designed to improve product accessibility for the nearly 43 million Americans stricken with arthritis--a core customer base for Advil.
The closure was reviewed by an independent panel and has earned praise from the Arthritis Foundation for its easy of opening and use. A flange design on top of the polypropylene cap makes it easier to turn. Consumers with limited use of their hands can also use a pen or pencil in the cap's U-shaped indent to give them more torquing power.
A similar cap had originally appeared on Advil's 72-count bottle. When the 72-count size was discontinued, the company decided to keep the "arthritis friendly" cap for its 165-count bottle.
Fran Sullivan, a Whitehall-Robins Healthcare spokesman, explains that this cap actually works better for the 165-count bottle. "This closure is not child resistant. The Consumer Product Safety Commission lets [drug companies] have one size in each product presentation [such as caplet, gel cap or tablet] that is non-child-resistant. They mandate that it not be in popular size, though. So we picked our 165-count because it's a little bit out of the norm," he says. And, for the people who might be taking Advil more often to relieve the pain of arthritis, the 165-count gives them better value.
When the company moved the cap to the 165-count size, it also changed to a wider high-density polyethylene bottle that's easier to grip. Only minor adjustments were needed to accommodate the new bottle/cap combination on the company's existing packaging line. Sullivan declined to identify the company's bottle and cap suppliers.
COPYRIGHT 2001 Stagnito Communications
COPYRIGHT 2003 Gale Group