Microspheres of Eudragit RL were developed foe delivery of albendazole specifically into the colon. The effects of polymer concentration, stirring rate, and concentration of emulsifier on particle size and drug loading were studied. A comparative in vitro drug release study of the optimized formulation was carried out in simulated colonic fluid, with and without 2% rat cecal material.
Drug delivery to the colon is beneficial not only for the oral delivery of proteins and peptide drugs (degraded by digestive enzymes of stomach and small intestine) but also for the delivery of low molecular weight compounds used to treat diseases associated with the colon or large intestine such as ulcerative colitis, diarrhea, and colon cancer. In addition, the colon has a long retention time and appears highly responsive to agents that enhance the absorption of poorly absorbed drugs. The approaches used in the colonic delivery of drugs include the use of prodrugs (1), pH-sensitive polymer coatings (2, 3), time-dependent formulations (4), bacterial-degradable coatings (5), time pH-controlled deliveries (6), and intestinal luminal pressure-controlled colon delivery capsules (7). In addition, the use of biodegradable polymers such as azopolymers and polysaccharides (e.g., pectins and dextrans) for colon targeting has also been described in the literature (8, 9).
Several publications have described drug-containing microspheres using the Eudragit (Rohm Pharma) series of polymers as the encapsulating materials (10, 11). The Eudragits are a family of polymers based on acrylic and methacrylic acids suitable for use in orally administered drug delivery systems. These polymers are available in various grades possessing a range of physicochemical properties. Some dissolve rapidly at clearly defined pH values, whereas two grades, Eudragit RL and RS, are insoluble in aqueous media but permeable, and as such have been shown to be suitable for sustained-release microencapsulated dosage forms. The permeability of Eudragit RL and RS to aqueous solutions results from the presence of quaternary ammonium groups in their structure; RL has a higher proportion of these groups and as such is more permeable than RS.
In recent years, microsphere dosage forms have gained increasing importance as oral controlled drug delivery systems. These systems present several advantages in comparison to unit dosage forms such as more predictable gastric emptying and less local irritation (12). Microsphere systems also minimize the possible intestinal retention of undigested polymer materials in chronic dosing (13).
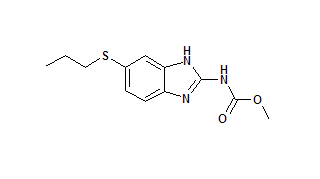
As part of a research program to investigate the controlled and targeted delivery of drugs to the colon, Eudragit RL polymer (ERLP) microspheres containing albendazole were prepared. Albendazole, used to treat parasites, was selected for colon delivery because the colon is the major site of residence for several types of worms (14), and only a very small amount of drug reaches the colon after oral administration (15). Eudragit polymers were selected to form the microspheres because they have been approved by the Food and Drug Administration and are widely used in the pharmaceutical industry. With this system, our aim was to avoid drug delivery in the upper gastrointestinal tract and target drug to the terminal ileum and colonic regions.
Materials and methods
Albendazole was obtained as a gift from S.A. Pharma (Sagar, India). Eudragit RL was obtained from Rohm GmbH Chemische Fabrik (Darmstadt, Germany). Liquid paraffin, methanol, petroleum ether, and other solvents were purchased from Hi-Media Chemical (Bombay, India). All other materials used in the dissolution studies were of analytical reagent grade and were used as received.
Preparation of Eudragit microspheres. The ERLP microspheres containing albendazole were prepared by the solvent evaporation method. In this procedure, a mixture of acetone and methanol in a ratio of 2:1 v/v was an effective solvent for dissolving both the drug and polymer. This organic solution was emulsified into liquid paraffin (70 mL) containing the emulsifier Span 80. The system was stirred continuously using a propeller stirrer at room temperature for 3-4 h to allow the complete evaporation of the solvent. The microspheres were collected and dried in a hot air oven at 50 [degrees]C. The effects of process variables such as drug-polymer ratio, stirring rate, and emulsifier concentration on the particle size and particle-size distribution of the microspheres, drug entrapment efficiency, and drug release were studied. ERLP microspheres were prepared using various drug-polymer ratios (i.e., 1:2, 1:3, 1:4, 1:5, and 1:6) while keeping the other two variables constant (i.e., stirring speed of 1000 rpm and emulsifier concentration 1.0% w/v). Similarly, microspheres were prepared at various stirring rates (i.e., 500, 1000, and 1500 rpm) while keeping the drug-polymer ratio at 1:4 and the emulsifier concentration at 1.0% w/v. Microspheres also were prepared using various emulsifier concentrations (i.e., 0.50, 0.75, 1.0, and 1.25% w/v) while keeping the drug-polymer ratio at 1:4 and the stirring speed at 1000 rpm.
Study of morphology and particle size. The morphology and appearance of microparticles were examined by scanning electron microscopy (SEM). The prepared microspheres were freeze-dried at -30 [degrees]C for 48 h and coated with gold palladium under an argon atmosphere for 150 s to achieve a 20-nm film (Sputter coater, SCD 004, BALTEC, Balzers, Furstentum, Liechtenstein). The coated samples were then examined using a scanning electron microscope (Jeol JSM-1600, Tokyo, Japan). The particle size of the prepared microspheres was determined by optical microscopy using a calibrated ocular micrometer (Leica, Germany).
High-performance liquid chromatography (HPLC) analysis of albendazole in microspheres and dissolution fluids. The quantitative determination of albendazole was performed by HPLC. The HPLC system used was a gradient HPLC (Shimadzu HPLC Class VP series) with two LC-10AT VP pumps, variable wavelength programmable UV/VIS Detector SPD-10A VP, a CTO-10AS VP column oven (Shimadzu), SLP-10A VP system controller (Shimadzu) and RP C-18 column (250 mm x 4.6 mm I.D.; particle size 5 [micro]m; YMC Inc., USA). The HPLC system was equipped with the Class-VP series software, version 5.03 (Shimadzu). The mobile phase used was a mixture of acetonitrile and triple distilled water containing 0.4% of triethylamine (pH adjusted to 3.6 with 5% orthophosphoric acid) in the ratio of 46:54. The filtered mobile phase was pumped at a flow rate of 1.2 mL/min. The column temperature was maintained at 40[degrees]C. UV detection at 254 nm detected the eluent and the data were acquired, stored, and analyzed with the installed Shimadzu software. A standard curve was constructed for albendazole in the range of 1-40 mg/mL, using mebendazole as the internal standard. A good linear relationship was observed between the concentration of albendazole and the ratio of the peak area of albendazole to that of mebendazole (the internal standard) with a high correlation coefficient (r = 0.9999). The required studies were carried out to estimate the precision and accuracy of this HPLC method of analyzing albendazole. The standard curve constructed as described above was used for estimating albendazole either in microspheres or in dissolution fluids.
Determination of drug content. An accurately weighed quantity of ERLP microspheres (equivalent to 100 mg of albendazole) was digested in 10 mL of methanol, and kept for 24 h for complete extraction of albendazole. The digested homogenate of microspheres was centrifuged at 3000 rpm for 5 min. The solution was filtered and the filtrate was subjected to HPLC analysis as described previously. Each determination was made in triplicate.
Drug release studies. An accurately weighed amount of ERLP microspheres, equivalent to 100 mg of albendazole, was added to 500 mL of dissolution medium and the release of albendazole from ERLP microspheres was investigated using the USP rotating paddle dissolution apparatus (Model DT-06, Erweka, Germany) at 100 rpm and 37[degrees]C. The simulation of gastrointestinal transit conditions was achieved by altering the pH of the dissolution medium at various time intervals. The pH of the dissolution medium was kept at 1.2 for 2 h with 0.1 N HCl. Then, 1.7 g of K[H.sub.2]P[O.sub.4] and 2.225 g of [Na.sub.2]HP[O.sub.4] x 2[H.sub.2]0 were added, adjusting the pH to 4.5 by adding 1.0 M NaOH. A release rate study was continued for another 2 h. After 4 h, the pH of the dissolution medium was adjusted to 7.0 and maintained for 24 h. The final volume in all cases was 500 mL. The samples were withdrawn from the dissolution medium at various time intervals using a pipette fitted with a microfilter, and the filtrate was subjected to HPLC analysis as described previously. All dissolution studies were performed in triplicate.
Preparation of 2% rat cecal material. Male albino rats weighing 105-115 g and maintained on a normal diet were used for the study. Six rats were asphyxiated using carbon dioxide. The abdomens were opened, the ceci were traced, ligated at both ends, dissected, and immediately transferred into pH 6.8 phosphate-buffered saline (PBS), previously bubbled with C[O.sub.2]. The cecal bags were opened and the contents were individually weighed, pooled, and then suspended in PBS to provide a 2% w/v dilution. Because the cecum is naturally anaerobic, all of these operations were carried out under C[O.sub.2] (16). The rats were cared for in accordance with institutional guidelines.
In vitro drug release study in the presence of colonic fluid containing 2% rat cecal material. The drug release studies were also carried out in simulated colonic fluid using a USP dissolution rate test apparatus (100 rpm, 37 [+ or -] 1 [degrees]C). A preweighed amount of microspheres was placed in the 200 mL of dissolution media (PBS pH 7.0) containing 2% w/v rat cecal material. The experiment was carried out with a continuous C[O.sub.2] supply into the dissolution medium. At different time intervals, the samples were withdrawn and replaced with fresh PBS. The experiment was continued for 24 h. The withdrawn samples were pipetted into a series of 10-mL volumetric flasks, filled to the 10-mL mark with PBS, and centrifuged. The supernatant was filtered through Whatman filter paper and the filtrate was subjected to HPLC analysis as described previously.
Stability studies. The selected formulation of microspheres was stored in amber-colored glass bottles at 4 [+ or -] 1 [degrees]C, 25 [+ or -] 1 [degrees]C, and 50 [+ or-] 1 [degrees]C for a period of 30 d and observed for any change in morphology of percentage residual drug content. Samples were analyzed for residual drug content at time intervals of 10 d for one month.
Results and discussion
The ERLP microspheres were prepared by a solvent evaporation method. A mixture of acetone and methanol (2:1 v/v) was found to be an effective solvent for the polymer and drug. It produced microparticles that were uniform in size, surface crosslinked, small, and almost spherical. An SEM photomicrograph of the microparticles is shown in Figure 1.
[FIGURE 1 OMITTED]
The effects of variables such as polymer concentration, stirring rate, and emulsifier concentrations on the particle size of microspheres were studied (see Table 1). The mean diameter of the ERLP microspheres at increasing ERLP concentrations (i.e., at drug-ERLP ratios from 1:2 to 1:6) increased from 135.3 to 163.4 [micro]m. This increase in particle size of the microspheres can be attributed to an increase in viscosity with increasing polymer concentrations, which resulted in larger emulsion droplets and finally in greater microsphere size (see Figure 2).
[FIGURE 2 OMITTED]
The mean diameters of microspheres prepared using various agitation speeds (i.e., 500, 1000, and 1500 rpm) were 158.6, 148.1, and 129.3 [micro]m, respectively. The dispersion of an acetic acid solution of the drug and ERLP into the droplets in the oil phase depended on the agitation speed of the systems. As agitation speed increased, the size of microspheres was reduced, but higher agitation speeds resulted in irregularly shaped microspheres. The mean diameter of the microspheres prepared using various concentrations of emulsifier (i.e., 0.50, 0.75, 1.00, and 1.25%),were 152.6, 147.1, 142.4, and 132.7 [micro]m, respectively. The more emulsifier added, the less irregular were the microspheres, and the size of the microspheres was reduced from 152.6 to 132.7 [micro]m. This appears to have resulted from a tightening of polymeric network, leading to microsphere shrinkage as the concentration of emulsifier is increased. The emulsifier is a surface-active agent. Among other properties, the emulsifier has the capability to stabilize the interface between the two phases. An increase in the level of emulsifier will allow it to stabilize a greater interfacial surface area, thus leading to smaller particle size.
The optimal drug loading of microspheres was determined using various processing variables (i.e., drug-ERLP ratio, stirring rate, and emulsifier concentration) (see Table I). The results indicate that the best drug loading percentage was observed at a drug-ERLP ratio of 1:4 (75.6%), a stirring rate of 1000 rpm (77.8%), and 1.0% w/v emulsifier concentration (73.8%). The higher percentage of drug loading from 73.8 to 77.8% indicates that ~25% of the drug may have leached out into the external phase during the emulsification process. The higher stirring rate prevented the coacervate droplets from coalescing and producing smaller microspheres, but it did not have any significant effect on drug loading.
The cumulative percent drug release curve of the ERLP microspheres showed the desired rate, i.e., no measurable drug release occurred up to 2 h in simulated gastric fluid (pH 1.2). At pH 4.5, up to 4 h, the drug release was insignificant (< 1%). In simulated intestinal fluid (pH 7.0) up to 24 h, the release of albendazole from ERLP microspheres decreased as the ERLP concentration increased, suggesting that drug release could be controlled by varying the ERLP concentration (see Figure 3). The results might also be explained by the fact that the higher ERLP content resulted in larger particles with proportionately less drug, so that the drug-polymer ratio was changed and thus release was reduced.
[FIGURE 3 OMITTED]
On the basis of the results of the study, the formulation of ERLP microspheres of albendazole was prepared under optimized conditions, i.e., a drug--ERLP ratio of 1:4, a stirring speed of 1000 rpm, and emulsifier concentration at 1.0% w/v.
These microspheres were spherical in shape and distributed in the size range of 200 to 250 [micro]m, with an average size of 220 [micro]m. The particles exhibited a smooth surface, as indicated in SEM photomicrographs (see Figure 1). The drug entrapment into the optimized microspheres was found to be 70-75%.
In the presence of 2% w/v cecal content, an ex vivo drug release study was performed in PBS (pH 7.0) using a USP dissolution test apparatus. The presence of rat cecal material in the dissolution medium increased the rate of drug release from the ERLP microspheres compared with the control. In vitro drug release in simulated colonic fluid without rat cecal material was 48.9%, but drug release in simulated colonic fluid with 2% rat cecal material after 24 h was 76.5%. The rat cecal material in the dissolution medium had increased the drug release from ERLP microspheres, which may be attributed to ERLP degradation by various anaerobic bacteria present in the caecum (see Figure 4). Hence, the drug release in the simulated colonic fluid with cecal content may be a result of the combined effects of diffusion and erosion.
[FIGURE 4 OMITTED]
The ERLP microspheres in the form of lyophilized powder were stored in glass bottles at 4 [+ or-] 1 [degrees]C, 25 [+ or 1] 1 [degrees]C, and 50 [+ or -] 1 [degrees]C for one month and evaluated for any change in the shape and structural integrity by microscopic examination and residual drug content. At 50 [+ or -] 1 [degrees]C, the microspheres lost their spherical shape (see Figure 5) indicating their instability at higher temperatures. Agglomerates of microspheres were formed after storage for one month at 50 [degrees]C, which may be attributed to polymer softening and fusion. The percentage residual drug content of microspheres was 98.2, 98.7, and 98.2% at 4, 25 and 50 [degrees]C, respectively after storage for 30 d.
[FIGURE 5 OMITTED]
Conclusion
The colon is an important site for the absorption and delivery of drugs used in the treatment of diseases associated with large intestine. Although the surface area of colon is small, it has long retention time. In this work, a formulation of albendazole that might be suitable for colonic delivery was prepared by entrapment into microspheres of Eudragit RL. Span 80, at a concentration of 1% w/v, was found to be a suitable emulsifying agent. Studies showed that the manipulation of polymer concentration and stirring rate influenced the drug loading and particle size of the microspheres and storage of Eudragit RL microspheres at low temperature is essential to retain their integrity. The approach described appears promising for the colonic delivery of drugs.
Acknowledgements
The authors greatly acknowledge S.A. Pharma, Sagar for the supply of albendazole as a gift sample. The authors wish to thank the director of IMMS, New Delhi, for providing the SEM facility.
References
(1.) T.N. Tozer et al., "Colon Specific Delivery of Dexamethasone from a Glucoside Prodrug in the Guinea Pig," Pharm. Res. 8 (4), 445-454 (1991).
(2.) M. Ashford et al., "An In Vivo Investigation into the Suitability of pH Dependent Polymers for Colonic Targeting," Int. J. Pharm. 95, 193-199 (1993).
(3.) M. Marvola et al., "Enteric Polymers as Binders and Coating Materials in Multiple-Unit Site-Specific Drug Delivery Systems," Eur. J. Pharm. Sci. 7 (3), 259-267 (1999).
(4.) C. Gazzaniga et al., "Time Dependent Oral Delivery System for the Colon Targeting," S.T.P. Pharm. Sci. 5, 83-88 (1995).
(5.) L.F. Siew, A.W. Basit, and J.M. Newton, "The Properties of Amylose-Ethylcellulose Films Cast from Organic-Based Solvents as Potential Coatings for Colonic Drug Delivery," Eur. J. Pharm. Sci. 11 (2), 133-139 (2000).
(6.) T. Ishibashi et al., "Design and Evaluation of a New Capsule-Type Dosage Form for Colon Targeted Delivery of Drugs," Int. J. Pharm. 168, 31-40 (1998).
(7.) Y. Yoshikawa et al., "A Dissolution Test for a Pressure-Controlled Colon Delivery Capsule: Rotating Beads Method," J. Pharm. Pharmacol. 51 (9), 979-989 (1999).
(8.) L. Hovgaard and H. Brondsted, "Dextran Hydrogels for Colon-Specific Drug Delivery," J. Control. Rel., 36, 159-166 (1995).
(9.) P.J. Watts and L. Illum, "Colonic Drug Delivery," Drug Dev. Ind. Pharm. 23, 893-913 (1997).
(10.) D. Babay, A. Hoffman, and S. Benita, "Design and Release Kinetic Pattern Evaluation of Indomethacin Microspheres," Biomaterials, 9, 482-488 (1988).
(11.) S. Benita, A. Hoffman, and M. Donbrow, "Microencapsulation of Paracetamol Using Polyacrylate Resins (Eudragit Retard), Kinetics of Drug Release and Evaluation of Kinetic Model," J. Pharm. Pharmacol., 37, 391-395 (1985).
(12.) M. Kilicarslan and T. Baykara, "The Effect of the Drug/Polymer Patio on the Properties of the Verapamil HCl Loaded Microspheres Intended for Oral Administration," Int. J. Pharm. 252 (1-2), 99-109 (2003).
(13.) J. Kramer and H. Blume, "Biopharmaceutical Aspects of Multiparticulates," in Multiparticulate Oral Drug Delivery, Y. Ghebre-Sellasie, Ed. (Marcel Dekker, New York, NY, 1994), pp. 307-332.
(14.) J.W. Tracy and L.T. Webster, Jr., "Drugs Used in the Chemotherapy of Helminthiasis," in Goodman and Gillman's The Pharmacological Basis of Therapeutics, J.G. Hardman and L.E. Limbird, Eds. 10th ed., (McGraw-Hill, Medical Publishing Division, New York, 2001), pp. 1125-1129.
(15.) Y.S.R. Krishnaiah et al., "Development of Colon Targeted Oral Guar Gum Matrix Tablets of Albendazole for the Treatment of Helminthiasis," Ind. J. Pharm. Sci., 65 (4), 378-385 (2003).
(16.) Y.S.R. Krishnaiah et al., "Guar Gum as a Carrier for Colon Specific Delivery; Influence of Metronidazole and Tinidazole on In Vitro Release of Albendazole from Guar Gum Ma trix Tablets," J. Pharm. Pharmaceut. Sci. 4 (3), 235-243 (2001).
Please rate this article.
On the Reader Service Card, circle a number:
348 Very useful and informative
349 Somewhat useful and informative
350 Not useful or informative
Your feedback is important to us.
Sunil K. Jain, Gopal Rai, D. K. Saraf, and G.P. Agrawal *
Sunil K. Jain and Gopal Rai are doctoral candidates and G.P. Agrawal, PhD, is a professor of pharmaceutics, all at the Department of Pharmaceutical Sciences, Dr. H.S. Gour University, Sagar, MP, 470 003, India, tel. +91 7582 221631, suniljain25in@yahoo.com. D.K. Saraf, PhD, is a reader of zoology, Department of Zoology, Dr. H.S. Gour University, Sagar, India.
* To whom all correspondence should be addressed.
COPYRIGHT 2004 Advanstar Communications, Inc.
COPYRIGHT 2005 Gale Group